Aquaporin 0 Modulates Lens Gap Junctions in the Presence of Lens-Specific Beaded Filament Proteins
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
ION CHANNEL DIVERSITY and CHARACTERIZATION
ION CHANNEL DIVERSITY and CHARACTERIZATION Voltage clamp techniques K channels Na channels Ca channels Ligand-gated channels Channelopathies OUTLINE Voltage clamp techniques whole cell, single channel, gating K channels Na channels Ca channels Cardiac AP Na nerve vs cardiac Ica: L vs T type; drugs (BayK, nitrendipine) Ito: inactivation, subtypes Kv1.4, Kv4.2/3, accessory subunits Ikr,Iks, Ikur (drugs dofetilide) IK1 – rectification Cardiac channelopathies (LQTS, SQTS, Brugada syndrome) Ligand-gated channels (AChR) Pancreatic beta cell channels (KATP, ICa) Voltage clamp techniques Capacitance currents (Ic) and ionic currents (Ii) are activated by rapid changes in membrane potential using voltage clamp Variable Vtest can be applied with voltage clamp 2.0 sec 40 duration 20 (10 sec between each pulse) 0 -20 mV test -40 V -60 -80 Simplified schematic of voltage clamp circuit Original patch clamp recordings (1981) Pflugers Arch 391: 85-100 Four modes of patch clamp technique High-throughput, automated patch clamp instruments EVOLUTION and Ion channel diversity Diversity of ion channels Example: nematode C. elegans 73 K channels (20 6 TM, 3 IRK, 50 TWIK) 89 ligand-gated channels (42 ACh, 37 inhibitory GABAA or glutamate, 10 excitatory glutamate) 5 voltage-gated Ca channels 6 chloride channels 24 gap junction channels (connexins) 22 mechanosensitive channels 6 cyclic-nucleotide gated channels 11 TRP-related channels Total: 236 channel subunit genes Origin of ion channel diversity 1) gene duplication & divergence 2) alternative mRNA splicing 3) -

Combined Pharmacological Administration of AQP1 Ion Channel
www.nature.com/scientificreports OPEN Combined pharmacological administration of AQP1 ion channel blocker AqB011 and water channel Received: 15 November 2018 Accepted: 13 August 2019 blocker Bacopaside II amplifes Published: xx xx xxxx inhibition of colon cancer cell migration Michael L. De Ieso 1, Jinxin V. Pei 1, Saeed Nourmohammadi1, Eric Smith 1,2, Pak Hin Chow1, Mohamad Kourghi1, Jennifer E. Hardingham 1,2 & Andrea J. Yool 1 Aquaporin-1 (AQP1) has been proposed as a dual water and cation channel that when upregulated in cancers enhances cell migration rates; however, the mechanism remains unknown. Previous work identifed AqB011 as an inhibitor of the gated human AQP1 cation conductance, and bacopaside II as a blocker of AQP1 water pores. In two colorectal adenocarcinoma cell lines, high levels of AQP1 transcript were confrmed in HT29, and low levels in SW480 cells, by quantitative PCR (polymerase chain reaction). Comparable diferences in membrane AQP1 protein levels were demonstrated by immunofuorescence imaging. Migration rates were quantifed using circular wound closure assays and live-cell tracking. AqB011 and bacopaside II, applied in combination, produced greater inhibitory efects on cell migration than did either agent alone. The high efcacy of AqB011 alone and in combination with bacopaside II in slowing HT29 cell motility correlated with abundant membrane localization of AQP1 protein. In SW480, neither agent alone was efective in blocking cell motility; however, combined application did cause inhibition of motility, consistent with low levels of membrane AQP1 expression. Bacopaside alone or combined with AqB011 also signifcantly impaired lamellipodial formation in both cell lines. Knockdown of AQP1 with siRNA (confrmed by quantitative PCR) reduced the efectiveness of the combined inhibitors, confrming AQP1 as a target of action. -

Src Regulation of Cx43 Phosphorylation and Gap Junction Turnover
biomolecules Article Src Regulation of Cx43 Phosphorylation and Gap Junction Turnover Joell L. Solan 1 and Paul D. Lampe 1,2,* 1 Translational Research Program, Fred Hutchinson Cancer Research Center, Seattle, WA 98109, USA; [email protected] 2 Department of Global Health, Pathobiology Program, University of Washington, Seattle, WA 98109, USA * Correspondence: [email protected] Received: 27 October 2020; Accepted: 22 November 2020; Published: 24 November 2020 Abstract: The gap junction protein Connexin43 (Cx43) is highly regulated by phosphorylation at over a dozen sites by probably at least as many kinases. This Cx43 “kinome” plays an important role in gap junction assembly and turnover. We sought to gain a better understanding of the interrelationship of these phosphorylation events particularly related to src activation and Cx43 turnover. Using state-of-the-art live imaging methods, specific inhibitors and many phosphorylation-status specific antibodies, we found phospho-specific domains in gap junction plaques and show evidence that multiple pathways of disassembly exist and can be regulated at the cellular and subcellular level. We found Src activation promotes formation of connexisomes (internalized gap junctions) in a process involving ERK-mediated phosphorylation of S279/282. Proteasome inhibition dramatically and rapidly restored gap junctions in the presence of Src and led to dramatic changes in the Cx43 phospho-profile including to increased Y247, Y265, S279/282, S365, and S373 phosphorylation. Lysosomal inhibition, on the other hand, nearly eliminated phosphorylation on Y247 and Y265 and reduced S368 and S373 while increasing S279/282 phosphorylation levels. We present a model of gap junction disassembly where multiple modes of disassembly are regulated by phosphorylation and can have differential effects on cellular signaling. -

Effects of Aquaporin 4 and Inward Rectifier
9-Experimental Surgery Effects of aquaporin 4 and inward rectifier potassium channel 4.1 on medullospinal edema after methylprednisolone treatment to suppress acute spinal cord injury in rats1 Ye LiI, Haifeng HuII, Jingchen LiuIII, Qingsan ZhuIV, Rui GuV IAssociate Professor, Department of Orthopaedics, China-Japan Union Hospital, Jilin University, Changchun, China. Conception, design, intellectual and scientific content of the study; acquisition of data; manuscript writing; critical revision. IIAttending Doctor, Department of Orthopaedics, China-Japan Union Hospital, Jilin University, Changchun, China. Acquisition of data, manuscript writing. IIIProfessor, Department of Orthopaedics, China-Japan Union Hospital, Jilin University, Changchun, China. Scientific content of the study, acquisition of data, manuscript writing. IVProfessor, Department of Orthopaedics, China-Japan Union Hospital, Jilin University, Changchun, China. Acquisition of data. VProfessor, Department of Orthopaedics, China-Japan Union Hospital, Jilin University, Changchun, China. Intellectual, scientific, conception and design of the study; critical revision. Abstract Purpose: To investigate the effects of aquaporin 4 (AQP4) and inward rectifier potassium channel 4.1 (Kir4.1) on medullospinal edema after treatment with methylprednisolone (MP) to suppress acute spinal cord injury (ASCI) in rats. Methods: Sprague Dawley rats were randomly divided into control, sham, ASCI, and MP- treated ASCI groups. After the induction of ASCI, we injected 30 mg/kg MP via the tail vein at various time points. The Tarlov scoring method was applied to evaluate neurological symptoms, and the wet–dry weights method was applied to measure the water content of the spinal cord. Results: The motor function score of the ASCI group was significantly lower than that of the sham group, and the spinal water content was significantly increased. -
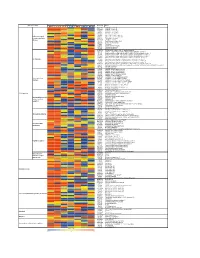
Supporting Figure 2
Normalized value Functional class Genbank Name Naive SC 1h SC 6h SC 24h WM 1h WM 6h WM 24h AB016161 GABA-B receptor 1d AF109405 GABA-B receptor 2a M35077 Dopamine-1A receptor S46131 Dopamine-1A receptor M84009 Dopamine receptor D4 U13368 Adrenergic receptor, alpha 1a G Protein-coupled M60654 Adrenergic receptor, alpha 1d receptors and their M64236 Tachykinin 1 receptor effectors AI229237 Opioid receptor-like Y11433 Pyrimidinergic receptor P2Y4 M64299 Adenosine A1 receptor E00001 Pro-insulin M29014 Insulin receptor precursor U35315 Serotonin receptor 2C S62043 Serotonin receptor 6 AF000368 Scn9a sodium channel, type IX, alpha polypeptide M27158 Kcna5 K+ voltage-gated channel, shaker-related subfamily, member 5 X17621 Kcna6 potassium voltage-gated channel, shaker-related, subfamily, member 6 X16476 Kcnb1 potassium voltage gated channel, Shab-related subfamily, member 1 M77482 Kcnb2 potassium voltage gated channel, Shab-related subfamily, member 2 S64320 Kcnd2 potassium voltage gated channel, Shal-related family, member 2 Ion channels X87635 Kcnj4 potassium inwardly-rectifying channel, subfamily J, member 4 D86039 Kcnj11 potassium inwardly-rectifying channel, subfamily J, member 11 X83581 Kcnj16 potassium inwardly-rectifying channel, subfamily J, member 16 AF073891 Kcnh5 potassium voltage-gated channel, subfamily H (eag-related), member 5 U69882 Kcnn2 potassium intermediate/small conductance calcium-activated channel, subfamily N, member 2 Z36944 Chloride channel 4-2 Z56277 Chloride channel 5 L08493 GABA-A receptor alpha-4 subunit X51992 -

Ubiquitination of Aquaporin-2 in the Kidney
Electrolytes & Blood Pressure 7:1-4, 2009 1 Review article 1) Ubiquitination of Aquaporin-2 in the Kidney Yu-Jung Lee, M.D. and Tae-Hwan Kwon, M.D. Department of Biochemistry and Cell Biology, School of Medicine, Kyungpook National University, Daegu, Korea Ubiquitination is known to be important for endocytosis and lysosomal degradation of aquaporin-2 (AQP2). Ubiquitin (Ub) is covalently attached to the lysine residue of the substrate proteins and activation and attach - ment of Ub to a target protein is mediated by the action of three enzymes (i.e., E1, E2, and E3). In particular, E3 Ub-protein ligases are known to have substrate specificity. This minireview will discuss the ubiquitination of AQP2 and identification of potential E3 Ub-protein ligases for 1-deamino-8-D-arginine vasopressin (dDAVP)-dependent AQP2 regulation. Key Words : kidney tubules, collecting; ubiquitination; vasopressins; aquaporin 2 The kidneys are responsible for the regulation of body This process produces concentrated urine and is essential water and electrolyte metabolism. Thus, understanding of for regulation of body water metabolism 6) . In contrast to the underlying mechanisms for renal water transport is the well-established signaling pathways for the vaso- critical. Water permeability along the nephron has already pressin-regulated AQP2 trafficking and up-regulation of been well characterized in the mammalian kidney 1) . AQP2 expression, the underlying mechanisms for AQP2 Approximately, 180 L/day of glomerular filtrate is gen- endocytosis and intracellular degradation of AQP2 protein erated in an adult human, more than 80-90% of the glomer- are unclear. So far, two hormones (prostaglandin E2 and ular filtrate is constitutively reabsorbed by the highly water dopamine) cause AQP2 internalization independent of permeable proximal tubules and descending thin limbs of S256 dephosphorylation 7, 8) . -

Engineering Biosynthetic Excitable Tissues from Unexcitable Cells for Electrophysiological and Cell Therapy Studies
ARTICLE Received 11 Nov 2010 | Accepted 5 Apr 2011 | Published 10 May 2011 DOI: 10.1038/ncomms1302 Engineering biosynthetic excitable tissues from unexcitable cells for electrophysiological and cell therapy studies Robert D. Kirkton1 & Nenad Bursac1 Patch-clamp recordings in single-cell expression systems have been traditionally used to study the function of ion channels. However, this experimental setting does not enable assessment of tissue-level function such as action potential (AP) conduction. Here we introduce a biosynthetic system that permits studies of both channel activity in single cells and electrical conduction in multicellular networks. We convert unexcitable somatic cells into an autonomous source of electrically excitable and conducting cells by stably expressing only three membrane channels. The specific roles that these expressed channels have on AP shape and conduction are revealed by different pharmacological and pacing protocols. Furthermore, we demonstrate that biosynthetic excitable cells and tissues can repair large conduction defects within primary 2- and 3-dimensional cardiac cell cultures. This approach enables novel studies of ion channel function in a reproducible tissue-level setting and may stimulate the development of new cell-based therapies for excitable tissue repair. 1 Department of Biomedical Engineering, Duke University, Durham, North Carolina 27708, USA. Correspondence and requests for materials should be addressed to N.B. (email: [email protected]). NatURE COMMUNicatiONS | 2:300 | DOI: 10.1038/ncomms1302 | www.nature.com/naturecommunications © 2011 Macmillan Publishers Limited. All rights reserved. ARTICLE NatUre cOMMUNicatiONS | DOI: 10.1038/ncomms1302 ll cells express ion channels in their membranes, but cells a b with a significantly polarized membrane that can undergo e 0 a transient all-or-none membrane depolarization (action A 1 potential, AP) are classified as ‘excitable cells’ . -

Ion Channels 3 1
r r r Cell Signalling Biology Michael J. Berridge Module 3 Ion Channels 3 1 Module 3 Ion Channels Synopsis Ion channels have two main signalling functions: either they can generate second messengers or they can function as effectors by responding to such messengers. Their role in signal generation is mainly centred on the Ca2 + signalling pathway, which has a large number of Ca2+ entry channels and internal Ca2+ release channels, both of which contribute to the generation of Ca2 + signals. Ion channels are also important effectors in that they mediate the action of different intracellular signalling pathways. There are a large number of K+ channels and many of these function in different + aspects of cell signalling. The voltage-dependent K (KV) channels regulate membrane potential and + excitability. The inward rectifier K (Kir) channel family has a number of important groups of channels + + such as the G protein-gated inward rectifier K (GIRK) channels and the ATP-sensitive K (KATP) + + channels. The two-pore domain K (K2P) channels are responsible for the large background K current. Some of the actions of Ca2 + are carried out by Ca2+-sensitive K+ channels and Ca2+-sensitive Cl − channels. The latter are members of a large group of chloride channels and transporters with multiple functions. There is a large family of ATP-binding cassette (ABC) transporters some of which have a signalling role in that they extrude signalling components from the cell. One of the ABC transporters is the cystic − − fibrosis transmembrane conductance regulator (CFTR) that conducts anions (Cl and HCO3 )and contributes to the osmotic gradient for the parallel flow of water in various transporting epithelia. -

An Update on Connexin Gap Junction and Hemichannels in Diabetic Retinopathy
International Journal of Molecular Sciences Review An Update on Connexin Gap Junction and Hemichannels in Diabetic Retinopathy Jorge González-Casanova 1 , Oliver Schmachtenberg 2, Agustín D. Martínez 3, Helmuth A. Sanchez 3, Paloma A. Harcha 3 and Diana Rojas-Gomez 4,* 1 Instituto de Ciencias Biomédicas, Facultad de Ciencias de la Salud, Universidad Autónoma de Chile, Santiago 8910060, Chile; [email protected] 2 Centro Interdisciplinario de Neurociencia de Valparaíso, Instituto de Biología, Facultad de Ciencias, Universidad de Valparaíso, Valparaíso 2360102, Chile; [email protected] 3 Centro Interdisciplinario de Neurociencia de Valparaíso, Instituto de Neurociencia, Facultad de Ciencias, Universidad de Valparaíso, Valparaíso 2360102, Chile; [email protected] (A.D.M.); [email protected] (H.A.S.); [email protected] (P.A.H.) 4 Escuela de Nutrición y Dietética, Facultad de Medicina, Universidad Andres Bello, Santiago 8370146, Chile * Correspondence: [email protected]; Tel.: +56-2-26618559 Abstract: Diabetic retinopathy (DR) is one of the main causes of vision loss in the working age popu- lation. It is characterized by a progressive deterioration of the retinal microvasculature, caused by long-term metabolic alterations inherent to diabetes, leading to a progressive loss of retinal integrity and function. The mammalian retina presents an orderly layered structure that executes initial but complex visual processing and analysis. Gap junction channels (GJC) forming electrical synapses are present in each retinal layer and contribute to the communication between different cell types. Citation: González-Casanova, J.; In addition, connexin hemichannels (HCs) have emerged as relevant players that influence diverse Schmachtenberg, O.; Martínez, A.D.; physiological and pathological processes in the retina. -

Microarray Analysis Reveals the Inhibition of Intestinal Expression Of
www.nature.com/scientificreports OPEN Microarray analysis reveals the inhibition of intestinal expression of nutrient transporters in piglets infected with porcine epidemic diarrhea virus Junmei Zhang1,3, Di Zhao1,3, Dan Yi1,3, Mengjun Wu1, Hongbo Chen1, Tao Wu1, Jia Zhou1, Peng Li1, Yongqing Hou1* & Guoyao Wu2 Porcine epidemic diarrhea virus (PEDV) infection can induce intestinal dysfunction, resulting in severe diarrhea and even death, but the mode of action underlying these viral efects remains unclear. This study determined the efects of PEDV infection on intestinal absorption and the expression of genes for nutrient transporters via biochemical tests and microarray analysis. Sixteen 7-day-old healthy piglets fed a milk replacer were randomly allocated to one of two groups. After 5-day adaption, piglets (n = 8/ group) were orally administrated with either sterile saline or PEDV (the strain from Yunnan province) 4.5 at 10 TCID50 (50% tissue culture infectious dose) per pig. All pigs were orally infused D-xylose (0.1 g/ kg BW) on day 5 post PEDV or saline administration. One hour later, jugular vein blood samples as well as intestinal samples were collected for further analysis. In comparison with the control group, PEDV infection increased diarrhea incidence, blood diamine oxidase activity, and iFABP level, while reducing growth and plasma D-xylose concentration in piglets. Moreover, PEDV infection altered plasma and jejunal amino acid profles, and decreased the expression of aquaporins and amino acid transporters (L-type amino acid -

Modulation of Voltage-Gated Potassium Channels by Phosphatidylinositol-4,5-Bisphosphate Marina Kasimova
Modulation of voltage-gated potassium channels by phosphatidylinositol-4,5-bisphosphate Marina Kasimova To cite this version: Marina Kasimova. Modulation of voltage-gated potassium channels by phosphatidylinositol-4,5- bisphosphate. Other. Université de Lorraine, 2014. English. NNT : 2014LORR0204. tel-01751176 HAL Id: tel-01751176 https://hal.univ-lorraine.fr/tel-01751176 Submitted on 29 Mar 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. AVERTISSEMENT Ce document est le fruit d'un long travail approuvé par le jury de soutenance et mis à disposition de l'ensemble de la communauté universitaire élargie. Il est soumis à la propriété intellectuelle de l'auteur. Ceci implique une obligation de citation et de référencement lors de l’utilisation de ce document. D'autre part, toute contrefaçon, plagiat, reproduction illicite encourt une poursuite pénale. Contact : [email protected] LIENS Code de la Propriété Intellectuelle. articles L 122. 4 Code de la Propriété Intellectuelle. articles L 335.2- L 335.10 http://www.cfcopies.com/V2/leg/leg_droi.php -

I REGENERATIVE MEDICINE APPROACHES to SPINAL CORD
REGENERATIVE MEDICINE APPROACHES TO SPINAL CORD INJURY A Dissertation Presented to The Graduate Faculty of The University of Akron In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Ashley Elizabeth Mohrman March 2017 i ABSTRACT Hundreds of thousands of people suffer from spinal cord injuries in the U.S.A. alone, with very few patients ever experiencing complete recovery. Complexity of the tissue and inflammatory response contribute to this lack of recovery, as the proper function of the central nervous system relies on its highly specific structural and spatial organization. The overall goal of this dissertation project is to study the central nervous system in the healthy and injured state so as to devise appropriate strategies to recover tissue homeostasis, and ultimately function, from an injured state. A specific spinal cord injury model, syringomyelia, was studied; this condition presents as a fluid filled cyst within the spinal cord. Molecular evaluation at three and six weeks post-injury revealed a large inflammatory response including leukocyte invasion, losses in neuronal transmission and signaling, and upregulation in important osmoregulators. These included osmotic stress regulating metabolites betaine and taurine, as well as the betaine/GABA transporter (BGT-1), potassium chloride transporter (KCC4), and water transporter aquaporin 1 (AQP1). To study cellular behavior in native tissue, adult neural stem cells from the subventricular niche were differentiated in vitro. These cells were tested under various culture conditions for cell phenotype preferences. A mostly pure (>80%) population of neural stem cells could be specified using soft, hydrogel substrates with a laminin coating and interferon-γ supplementation.