Paxilline, a Closed BK Channel Blocker Expression Produces a 13-Mv Or 32-Mv Rightward Shift of Voltage Neces- Yu Zhou, Christopher J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
ION CHANNEL DIVERSITY and CHARACTERIZATION
ION CHANNEL DIVERSITY and CHARACTERIZATION Voltage clamp techniques K channels Na channels Ca channels Ligand-gated channels Channelopathies OUTLINE Voltage clamp techniques whole cell, single channel, gating K channels Na channels Ca channels Cardiac AP Na nerve vs cardiac Ica: L vs T type; drugs (BayK, nitrendipine) Ito: inactivation, subtypes Kv1.4, Kv4.2/3, accessory subunits Ikr,Iks, Ikur (drugs dofetilide) IK1 – rectification Cardiac channelopathies (LQTS, SQTS, Brugada syndrome) Ligand-gated channels (AChR) Pancreatic beta cell channels (KATP, ICa) Voltage clamp techniques Capacitance currents (Ic) and ionic currents (Ii) are activated by rapid changes in membrane potential using voltage clamp Variable Vtest can be applied with voltage clamp 2.0 sec 40 duration 20 (10 sec between each pulse) 0 -20 mV test -40 V -60 -80 Simplified schematic of voltage clamp circuit Original patch clamp recordings (1981) Pflugers Arch 391: 85-100 Four modes of patch clamp technique High-throughput, automated patch clamp instruments EVOLUTION and Ion channel diversity Diversity of ion channels Example: nematode C. elegans 73 K channels (20 6 TM, 3 IRK, 50 TWIK) 89 ligand-gated channels (42 ACh, 37 inhibitory GABAA or glutamate, 10 excitatory glutamate) 5 voltage-gated Ca channels 6 chloride channels 24 gap junction channels (connexins) 22 mechanosensitive channels 6 cyclic-nucleotide gated channels 11 TRP-related channels Total: 236 channel subunit genes Origin of ion channel diversity 1) gene duplication & divergence 2) alternative mRNA splicing 3) -

Src Regulation of Cx43 Phosphorylation and Gap Junction Turnover
biomolecules Article Src Regulation of Cx43 Phosphorylation and Gap Junction Turnover Joell L. Solan 1 and Paul D. Lampe 1,2,* 1 Translational Research Program, Fred Hutchinson Cancer Research Center, Seattle, WA 98109, USA; [email protected] 2 Department of Global Health, Pathobiology Program, University of Washington, Seattle, WA 98109, USA * Correspondence: [email protected] Received: 27 October 2020; Accepted: 22 November 2020; Published: 24 November 2020 Abstract: The gap junction protein Connexin43 (Cx43) is highly regulated by phosphorylation at over a dozen sites by probably at least as many kinases. This Cx43 “kinome” plays an important role in gap junction assembly and turnover. We sought to gain a better understanding of the interrelationship of these phosphorylation events particularly related to src activation and Cx43 turnover. Using state-of-the-art live imaging methods, specific inhibitors and many phosphorylation-status specific antibodies, we found phospho-specific domains in gap junction plaques and show evidence that multiple pathways of disassembly exist and can be regulated at the cellular and subcellular level. We found Src activation promotes formation of connexisomes (internalized gap junctions) in a process involving ERK-mediated phosphorylation of S279/282. Proteasome inhibition dramatically and rapidly restored gap junctions in the presence of Src and led to dramatic changes in the Cx43 phospho-profile including to increased Y247, Y265, S279/282, S365, and S373 phosphorylation. Lysosomal inhibition, on the other hand, nearly eliminated phosphorylation on Y247 and Y265 and reduced S368 and S373 while increasing S279/282 phosphorylation levels. We present a model of gap junction disassembly where multiple modes of disassembly are regulated by phosphorylation and can have differential effects on cellular signaling. -
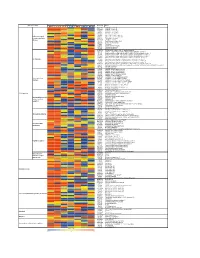
Supporting Figure 2
Normalized value Functional class Genbank Name Naive SC 1h SC 6h SC 24h WM 1h WM 6h WM 24h AB016161 GABA-B receptor 1d AF109405 GABA-B receptor 2a M35077 Dopamine-1A receptor S46131 Dopamine-1A receptor M84009 Dopamine receptor D4 U13368 Adrenergic receptor, alpha 1a G Protein-coupled M60654 Adrenergic receptor, alpha 1d receptors and their M64236 Tachykinin 1 receptor effectors AI229237 Opioid receptor-like Y11433 Pyrimidinergic receptor P2Y4 M64299 Adenosine A1 receptor E00001 Pro-insulin M29014 Insulin receptor precursor U35315 Serotonin receptor 2C S62043 Serotonin receptor 6 AF000368 Scn9a sodium channel, type IX, alpha polypeptide M27158 Kcna5 K+ voltage-gated channel, shaker-related subfamily, member 5 X17621 Kcna6 potassium voltage-gated channel, shaker-related, subfamily, member 6 X16476 Kcnb1 potassium voltage gated channel, Shab-related subfamily, member 1 M77482 Kcnb2 potassium voltage gated channel, Shab-related subfamily, member 2 S64320 Kcnd2 potassium voltage gated channel, Shal-related family, member 2 Ion channels X87635 Kcnj4 potassium inwardly-rectifying channel, subfamily J, member 4 D86039 Kcnj11 potassium inwardly-rectifying channel, subfamily J, member 11 X83581 Kcnj16 potassium inwardly-rectifying channel, subfamily J, member 16 AF073891 Kcnh5 potassium voltage-gated channel, subfamily H (eag-related), member 5 U69882 Kcnn2 potassium intermediate/small conductance calcium-activated channel, subfamily N, member 2 Z36944 Chloride channel 4-2 Z56277 Chloride channel 5 L08493 GABA-A receptor alpha-4 subunit X51992 -

Engineering Biosynthetic Excitable Tissues from Unexcitable Cells for Electrophysiological and Cell Therapy Studies
ARTICLE Received 11 Nov 2010 | Accepted 5 Apr 2011 | Published 10 May 2011 DOI: 10.1038/ncomms1302 Engineering biosynthetic excitable tissues from unexcitable cells for electrophysiological and cell therapy studies Robert D. Kirkton1 & Nenad Bursac1 Patch-clamp recordings in single-cell expression systems have been traditionally used to study the function of ion channels. However, this experimental setting does not enable assessment of tissue-level function such as action potential (AP) conduction. Here we introduce a biosynthetic system that permits studies of both channel activity in single cells and electrical conduction in multicellular networks. We convert unexcitable somatic cells into an autonomous source of electrically excitable and conducting cells by stably expressing only three membrane channels. The specific roles that these expressed channels have on AP shape and conduction are revealed by different pharmacological and pacing protocols. Furthermore, we demonstrate that biosynthetic excitable cells and tissues can repair large conduction defects within primary 2- and 3-dimensional cardiac cell cultures. This approach enables novel studies of ion channel function in a reproducible tissue-level setting and may stimulate the development of new cell-based therapies for excitable tissue repair. 1 Department of Biomedical Engineering, Duke University, Durham, North Carolina 27708, USA. Correspondence and requests for materials should be addressed to N.B. (email: [email protected]). NatURE COMMUNicatiONS | 2:300 | DOI: 10.1038/ncomms1302 | www.nature.com/naturecommunications © 2011 Macmillan Publishers Limited. All rights reserved. ARTICLE NatUre cOMMUNicatiONS | DOI: 10.1038/ncomms1302 ll cells express ion channels in their membranes, but cells a b with a significantly polarized membrane that can undergo e 0 a transient all-or-none membrane depolarization (action A 1 potential, AP) are classified as ‘excitable cells’ . -

Ion Channels 3 1
r r r Cell Signalling Biology Michael J. Berridge Module 3 Ion Channels 3 1 Module 3 Ion Channels Synopsis Ion channels have two main signalling functions: either they can generate second messengers or they can function as effectors by responding to such messengers. Their role in signal generation is mainly centred on the Ca2 + signalling pathway, which has a large number of Ca2+ entry channels and internal Ca2+ release channels, both of which contribute to the generation of Ca2 + signals. Ion channels are also important effectors in that they mediate the action of different intracellular signalling pathways. There are a large number of K+ channels and many of these function in different + aspects of cell signalling. The voltage-dependent K (KV) channels regulate membrane potential and + excitability. The inward rectifier K (Kir) channel family has a number of important groups of channels + + such as the G protein-gated inward rectifier K (GIRK) channels and the ATP-sensitive K (KATP) + + channels. The two-pore domain K (K2P) channels are responsible for the large background K current. Some of the actions of Ca2 + are carried out by Ca2+-sensitive K+ channels and Ca2+-sensitive Cl − channels. The latter are members of a large group of chloride channels and transporters with multiple functions. There is a large family of ATP-binding cassette (ABC) transporters some of which have a signalling role in that they extrude signalling components from the cell. One of the ABC transporters is the cystic − − fibrosis transmembrane conductance regulator (CFTR) that conducts anions (Cl and HCO3 )and contributes to the osmotic gradient for the parallel flow of water in various transporting epithelia. -

An Update on Connexin Gap Junction and Hemichannels in Diabetic Retinopathy
International Journal of Molecular Sciences Review An Update on Connexin Gap Junction and Hemichannels in Diabetic Retinopathy Jorge González-Casanova 1 , Oliver Schmachtenberg 2, Agustín D. Martínez 3, Helmuth A. Sanchez 3, Paloma A. Harcha 3 and Diana Rojas-Gomez 4,* 1 Instituto de Ciencias Biomédicas, Facultad de Ciencias de la Salud, Universidad Autónoma de Chile, Santiago 8910060, Chile; [email protected] 2 Centro Interdisciplinario de Neurociencia de Valparaíso, Instituto de Biología, Facultad de Ciencias, Universidad de Valparaíso, Valparaíso 2360102, Chile; [email protected] 3 Centro Interdisciplinario de Neurociencia de Valparaíso, Instituto de Neurociencia, Facultad de Ciencias, Universidad de Valparaíso, Valparaíso 2360102, Chile; [email protected] (A.D.M.); [email protected] (H.A.S.); [email protected] (P.A.H.) 4 Escuela de Nutrición y Dietética, Facultad de Medicina, Universidad Andres Bello, Santiago 8370146, Chile * Correspondence: [email protected]; Tel.: +56-2-26618559 Abstract: Diabetic retinopathy (DR) is one of the main causes of vision loss in the working age popu- lation. It is characterized by a progressive deterioration of the retinal microvasculature, caused by long-term metabolic alterations inherent to diabetes, leading to a progressive loss of retinal integrity and function. The mammalian retina presents an orderly layered structure that executes initial but complex visual processing and analysis. Gap junction channels (GJC) forming electrical synapses are present in each retinal layer and contribute to the communication between different cell types. Citation: González-Casanova, J.; In addition, connexin hemichannels (HCs) have emerged as relevant players that influence diverse Schmachtenberg, O.; Martínez, A.D.; physiological and pathological processes in the retina. -

Localization of the Kv1.5 K+ Channel Protein in Explanted Cardiac Tissue
Localization of the Kv1.5 K+ channel protein in explanted cardiac tissue. D J Mays, … , L H Philipson, M M Tamkun J Clin Invest. 1995;96(1):282-292. https://doi.org/10.1172/JCI118032. Research Article The cloned Kv1.5 K+ channel displays similar kinetics and pharmacology to a delayed rectifier channel found in atrial myocytes. To determine whether the Kv1.5 isoform plays a role in the cardiac action potential, it is necessary to confirm the expression of this channel in cardiac myocytes. Using antibodies directed against two distinct channel epitopes, the Kv1.5 isoform was localized in human atrium and ventricle. Kv1.5 was highly localized at intercalated disk regions as determined by colocalization with connexin and N-cadherin specific antibodies. While both antichannel antibodies localized the Kv1.5 protein in cardiac myocytes, only the NH2-terminal antibodies stained vascular smooth muscle. The selective staining of vasculature by this antiserum suggests that epitope accessibility, and perhaps channel structure, varies between cardiac and vascular myocytes. Kv1.5 expression was localized less in newborn tissue, with punctate antibody staining dispersed on the myocyte surface. This increasing organization with age was similar to that observed for connexin. Future work will address whether altered K+ channel localization is associated with cardiac disease in addition to changing with development. Find the latest version: https://jci.me/118032/pdf Localization of the Kv1.5 K+ Channel Protein in Explanted Cardiac Tissue D. J. Mays,* J. M. Foose,* L. H. Philipson,11 and M. M. Tamkun*§ Departments of *Molecular Physiology and Biophysics, tPediatrics, and OPharmacology, Vanderbilt University School of Medicine, Nashville, Tennessee 37232; I1Department of Medicine, University of Chicago, Chicago, Illinois 92101 Abstract expression system of choice. -

UC Berkeley UC Berkeley Electronic Theses and Dissertations
UC Berkeley UC Berkeley Electronic Theses and Dissertations Title The Effects of Neurosteroids, such as Pregnenolone Sulfate and its receptor, TrpM3 in the Retina. Permalink https://escholarship.org/uc/item/04d8607f Author Webster, Corey Michael Publication Date 2019 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California The Effects of Neurosteroids, such as Pregnenolone Sulfate, and its receptor, TrpM3 in the Retina. By Corey Webster A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Molecular and Cell Biology in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Marla Feller, Chair Professor Diana Bautista Professor Daniella Kaufer Professor Stephan Lammel Fall 2019 The Effects of Neurosteroids, such as Pregnenolone Sulfate, and its receptor, TrpM3 in the Retina. Copyright 2019 by Corey Webster Abstract The Effects of Neurosteroids, such as Pregnenolone Sulfate, and its receptor, TrpM3 in the Retina. by Corey M. Webster Doctor of Philosophy in Molecular and Cell Biology University of California, Berkeley Professor Marla Feller, Chair Pregnenolone sulfate (PregS) is the precursor to all steroid hormones and is produced in neurons in an activity dependent manner. Studies have shown that PregS production is upregulated during certain critical periods of development, such as in the first year of life in humans, during adolescence, and during pregnancy. Conversely, PregS is decreased during aging, as well as in several neurodevelopmental and neurodegenerative conditions. There are several known targets of PregS, such as a positive allosteric modulator NMDA receptors, sigma1 receptor, and as a negative allosteric modulator of GABA-A receptors. -

The Drosophila Innexin Multiprotein Family of Gap Junction Proteins
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Chemistry & Biology, Vol. 12, 515–526, May, 2005, ©2005 Elsevier Ltd All rights reserved. DOI 10.1016/j.chembiol.2005.02.013 Intercellular Communication: Review the Drosophila Innexin Multiprotein Family of Gap Junction Proteins Reinhard Bauer, Birgit Löer, Katinka Ostrowski, with the morphological description of gap junctions as Julia Martini, Andy Weimbs, Hildegard Lechner, having a “nexus” structure. Connexins comprise a and Michael Hoch* multigene family of integral membrane proteins, with 20 Institute of Molecular Physiology and Connexin isoforms identified in mice and 21 in humans Developmental Biology [9]. These proteins are characterized by two extracellu- University of Bonn lar domains, four membrane-spanning domains, and Poppelsdorfer Schloss three cytoplasmic domains, consisting of an intracellu- 53115 Bonn lar loop, and amino- and carboxy termini. Six Connexin Germany transmembrane protein units form a hemichannel, which is termed the Connexon (see [10] for review). In mam- mals, two hemichannels form a gap junction channel, Summary with each hemichannel provided by one of the two neighboring cells. These two hemichannels dock head- Gap junctions belong to the most conserved cellular to-head in the extracellular space to form a tightly structures in multicellular organisms, from Hydra to sealed, double-membrane intercellular gap junction man. They contain tightly packed clusters of hydro- channel. Gap junctions allow diffusional exchange of philic membrane channels connecting the cytoplasms ions, such as Ca2+, and metabolites, such as inositol of adjacent cells, thus allowing direct communication phosphates and cyclic nucleotides (see [11] and [12] of cells and tissues through the diffusion of ions, me- for review). -

Aquaporin 0 Modulates Lens Gap Junctions in the Presence of Lens-Specific Beaded Filament Proteins
Lens Aquaporin 0 Modulates Lens Gap Junctions in the Presence of Lens-Specific Beaded Filament Proteins Sindhu Kumari,1 Junyuan Gao,1 Richard T. Mathias,1,2 Xiurong Sun,1 Amizhdini Eswaramoorthy,1 Nicholas Browne,1 Nigel Zhang,1 and Kulandaiappan Varadaraj1,2 1Department of Physiology and Biophysics, Stony Brook University, Stony Brook, New York, United States 2SUNY Eye Institute, Syracuse, New York, United States Correspondence: Kulandaiappan PURPOSE. The objective of this study was to understand the molecular and physiologic Varadaraj, Department of Physiology mechanisms behind the lens cataract differences in Aquaporin 0-knockout-Heterozygous and Biophysics, BST-6, Room # 165A, (AQP0-Htz) mice developed in C57 and FVB (lacks beaded filaments [BFs]) strains. School of Medicine, Stony Brook University, NY 11794-8661, USA; METHODS. Lens transparency was studied using dark field light microscopy. Water permeability kulandaiappan.varadaraj@ (Pf) was measured in fiber cell membrane vesicles. Western blotting/immunostaining was stonybrook.edu. performed to verify expression of BF proteins and connexins. Microelectrode-based intact Submitted: May 2, 2017 lens intracellular impedance was measured to determine gap junction (GJ) coupling Accepted: October 23, 2017 resistance. Lens intracellular hydrostatic pressure (HP) was determined using a microelec- trode/manometer system. Citation: Kumari S, Gao J, Mathias RT, et al. Aquaporin 0 modulates lens gap RESULTS. Lens opacity and spherical aberration were more distinct in AQP0-Htz lenses from junctions in the presence of lens- FVB than C57 strains. In either background, compared to wild type (WT), AQP0-Htz lenses specific beaded filament proteins. showed decreased Pf (approximately 50%), which was restored by transgenic expression of Invest Ophthalmol Vis Sci. -

The Relevance of Aquaporins for the Physiology, Pathology, and Aging of the Female Reproductive System in Mammals
cells Review The Relevance of Aquaporins for the Physiology, Pathology, and Aging of the Female Reproductive System in Mammals Paweł Kordowitzki 1,2 , Wiesława Kranc 3, Rut Bryl 3, Bartosz Kempisty 3,4,5, Agnieszka Skowronska 6 and Mariusz T. Skowronski 1,* 1 Department of Basic and Preclinical Sciences, Institute for Veterinary Medicine, Nicolaus Copernicus University, 87-100 Torun, Poland; [email protected] 2 Institute of Animal Reproduction and Food Research of Polish Academy of Sciences, 10-243 Olsztyn, Poland 3 Department of Anatomy, Poznan University of Medical Sciences, 60-781 Poznan, Poland; [email protected] (W.K.); [email protected] (R.B.); [email protected] (B.K.) 4 Department of Histology and Embryology, Poznan University of Medical Sciences, 60-781 Poznan, Poland 5 Department of Veterinary Surgery, Institute for Veterinary Medicine, Nicolaus Copernicus University, 87-100 Torun, Poland 6 Department of Human Physiology and Pathophysiology, School of Medicine, Collegium Medicum, University of Warmia and Mazury, Warszawska Street 30, 10-082 Olsztyn, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-56-611-2231 Received: 27 October 2020; Accepted: 29 November 2020; Published: 1 December 2020 Abstract: Aquaporins constitute a group of water channel proteins located in numerous cell types. These are pore-forming transmembrane proteins, which mediate the specific passage of water molecules through membranes. It is well-known that water homeostasis plays a crucial role in different reproductive processes, e.g., oocyte transport, hormonal secretion, completion of successful fertilization, blastocyst formation, pregnancy, and birth. Further, aquaporins are involved in the process of spermatogenesis, and they have been reported to be involved during the storage of spermatozoa. -

Direct Immunogold Labeling of Aquaporin-4 in Square Arrays of Astrocyte and Ependymocyte Plasma Membranes in Rat Brain and Spinal Cord
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 11981–11986, September 1998 Neurobiology Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord i JOHN E. RASH*†‡§,THOMAS YASUMURA*, C. SUE HUDSON*, PETER AGRE¶, AND SØREN NIELSEN *Department of Anatomy and Neurobiology, †Program in Molecular, Cellular, and Integrative Neurosciences, and ‡Program in Cell and Molecular Biology, Colorado State University, Fort Collins, CO 80523; ¶Departments of Biological Chemistry and Medicine, Johns Hopkins University School of Medicine; and iDepartment of Cell Biology, Institute of Anatomy, University of Aarhus, Aarhus, Denmark Communicated by Thomas S. Reese, National Institutes of Health, Bethesda, MD, August 13, 1998 (received for review March 1, 1998) ABSTRACT Aquaporin (AQP) water channels are abun- the glia limitans and that surround capillaries (3). AQP4 is dant in the brain and spinal cord, where AQP1 and AQP4 are especially abundant in astrocytes and ependymocytes in os- believed to play major roles in water metabolism and osmo- mosensory areas, including the supraoptic nucleus and sub- regulation. Immunocytochemical analysis of the brain re- fornical organ (3). These sites suggest a role for AQP4 in water cently revealed that AQP4 has a highly polarized distribution, transport between the brain parenchyma, cerebrospinal fluid, with marked expression in astrocyte end-feet that surround and blood, as well as a role in osmoregulation. capillaries and form the glia limitans; however, the structural Naturally occurring membranes enriched in AQP1 contain organization of AQP4 has remained unknown. In freeze- intramembrane particles (IMPs). Nevertheless, membrane re- fracture replicas, astrocyte end-feet contain abundant square constitution of AQP1 at high concentration yielded highly arrays of intramembrane particles that parallel the distribu- ordered two-dimensional crystalline lattices of AQP1, with a tion of AQP4.