Auxiliary Trafficking Subunit GJA1-20K Protects Connexin-43 from Degradation and Limits Ventricular Arrhythmias
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Post-Mortem Whole-Exome Analysis in a Large Sudden Infant Death Syndrome Cohort with a Focus on Cardiovascular and Metabolic Genetic Diseases
European Journal of Human Genetics (2017) 25, 404–409 & 2017 Macmillan Publishers Limited, part of Springer Nature. All rights reserved 1018-4813/17 www.nature.com/ejhg ARTICLE Post-mortem whole-exome analysis in a large sudden infant death syndrome cohort with a focus on cardiovascular and metabolic genetic diseases Jacqueline Neubauer*,1, Maria Rita Lecca2, Giancarlo Russo2, Christine Bartsch3, Argelia Medeiros-Domingo4, Wolfgang Berger5,6,7 and Cordula Haas1 Sudden infant death syndrome (SIDS) is described as the sudden and unexplained death of an apparently healthy infant younger than one year of age. Genetic studies indicate that up to 35% of SIDS cases might be explained by familial or genetic diseases such as cardiomyopathies, ion channelopathies or metabolic disorders that remained undetected during conventional forensic autopsy procedures. Post-mortem genetic testing by using massive parallel sequencing (MPS) approaches represents an efficient and rapid tool to further investigate unexplained death cases and might help to elucidate pathogenic genetic variants and mechanisms in cases without a conclusive cause of death. In this study, we performed whole-exome sequencing (WES) in 161 European SIDS infants with focus on 192 genes associated with cardiovascular and metabolic diseases. Potentially causative variants were detected in 20% of the SIDS cases. The majority of infants had variants with likely functional effects in genes associated with channelopathies (9%), followed by cardiomyopathies (7%) and metabolic diseases (1%). Although lethal arrhythmia represents the most plausible and likely cause of death, the majority of SIDS cases still remains elusive and might be explained by a multifactorial etiology, triggered by a combination of different genetic and environmental risk factors. -
ION CHANNEL DIVERSITY and CHARACTERIZATION
ION CHANNEL DIVERSITY and CHARACTERIZATION Voltage clamp techniques K channels Na channels Ca channels Ligand-gated channels Channelopathies OUTLINE Voltage clamp techniques whole cell, single channel, gating K channels Na channels Ca channels Cardiac AP Na nerve vs cardiac Ica: L vs T type; drugs (BayK, nitrendipine) Ito: inactivation, subtypes Kv1.4, Kv4.2/3, accessory subunits Ikr,Iks, Ikur (drugs dofetilide) IK1 – rectification Cardiac channelopathies (LQTS, SQTS, Brugada syndrome) Ligand-gated channels (AChR) Pancreatic beta cell channels (KATP, ICa) Voltage clamp techniques Capacitance currents (Ic) and ionic currents (Ii) are activated by rapid changes in membrane potential using voltage clamp Variable Vtest can be applied with voltage clamp 2.0 sec 40 duration 20 (10 sec between each pulse) 0 -20 mV test -40 V -60 -80 Simplified schematic of voltage clamp circuit Original patch clamp recordings (1981) Pflugers Arch 391: 85-100 Four modes of patch clamp technique High-throughput, automated patch clamp instruments EVOLUTION and Ion channel diversity Diversity of ion channels Example: nematode C. elegans 73 K channels (20 6 TM, 3 IRK, 50 TWIK) 89 ligand-gated channels (42 ACh, 37 inhibitory GABAA or glutamate, 10 excitatory glutamate) 5 voltage-gated Ca channels 6 chloride channels 24 gap junction channels (connexins) 22 mechanosensitive channels 6 cyclic-nucleotide gated channels 11 TRP-related channels Total: 236 channel subunit genes Origin of ion channel diversity 1) gene duplication & divergence 2) alternative mRNA splicing 3) -

Cardiomyopathy
UNIVERSITY OF MINNESOTA PHYSICIANS OUTREACH LABS Submit this form along with the appropriate MOLECULAR DIAGNOSTICS (612) 273-8445 Molecular requisition (Molecular Diagnostics or DATE: TIME COLLECTED: PCU/CLINIC: Molecular NGS Oncology). AM PM PATIENT IDENTIFICATION DIAGNOSIS (Dx) / DIAGNOSIS CODES (ICD-9) - OUTPATIENTS ONLY SPECIMEN TYPE: o Blood (1) (2) (3) (4) PLEASE COLLECT 5-10CC IN ACD-A OR EDTA TUBE ORDERING PHYSICIAN NAME AND PHONE NUMBER: Tests can be ordered as a full panel, or by individual gene(s). Please contact the genetic counselor with any questions at 612-624-8948 or by pager at 612-899-3291. _______________________________________________ Test Ordered- EPIC: Next generation sequencing(Next Gen) Sunquest: NGS Brugada syndrome DMD Aortopathy Full panel DNAJC19 SCN5A DSP Full panel GPD1L Cardiomyopathy, familial MYH11 CACNA1C hypertrophic ACTA2 SCN1B Full panel MYLK KCNE3 ANKRD1 FBN2 SCN3B JPH2 SLC2A10 HCN4 PRKAG2 COL5A2 MYH7 COL5A1 MYL2 COL3A1 Cardiomyopathy ACTC1 CBS Cardiomyopathy, dilated CSRP3 SMAD3 Full panel TNNC1 TGFBR1 LDB3 MYH6 TGFBR2 LMNA VCL FBN1 ACTN2 MYOZ2 Arrhythmogenic right ventricular DSG2 PLN dysplasia NEXN CALR3 TNNT2 TNNT2 NEXN Full panel RBM20 TPM1 TGFB3 SCN5A MYBPC3 DSG2 MYH6 TNNI3 DSC2 TNNI3 MYL3 JUP TTN TTN RYR2 BAG3 MYLK2 TMEM43 DES Cardiomyopathy, familial DSP CRYAB EYA4 restrictive PKP2 Full panel Atrial fibrillation LAMA4 MYPN TNNI3 SGCD MYPN TNNT2 Full panel CSRP3 SCN5A MYBPC3 GJA5 TCAP ABCC9 ABCC9 SCN1B PLN SCN2B ACTC1 KCNQ1 MYH7 KCNE2 TMPO NPPA VCL NPPA TPM1 KCNA5 TNNC1 KCNJ2 GATAD1 4/1/2014 -

Src Regulation of Cx43 Phosphorylation and Gap Junction Turnover
biomolecules Article Src Regulation of Cx43 Phosphorylation and Gap Junction Turnover Joell L. Solan 1 and Paul D. Lampe 1,2,* 1 Translational Research Program, Fred Hutchinson Cancer Research Center, Seattle, WA 98109, USA; [email protected] 2 Department of Global Health, Pathobiology Program, University of Washington, Seattle, WA 98109, USA * Correspondence: [email protected] Received: 27 October 2020; Accepted: 22 November 2020; Published: 24 November 2020 Abstract: The gap junction protein Connexin43 (Cx43) is highly regulated by phosphorylation at over a dozen sites by probably at least as many kinases. This Cx43 “kinome” plays an important role in gap junction assembly and turnover. We sought to gain a better understanding of the interrelationship of these phosphorylation events particularly related to src activation and Cx43 turnover. Using state-of-the-art live imaging methods, specific inhibitors and many phosphorylation-status specific antibodies, we found phospho-specific domains in gap junction plaques and show evidence that multiple pathways of disassembly exist and can be regulated at the cellular and subcellular level. We found Src activation promotes formation of connexisomes (internalized gap junctions) in a process involving ERK-mediated phosphorylation of S279/282. Proteasome inhibition dramatically and rapidly restored gap junctions in the presence of Src and led to dramatic changes in the Cx43 phospho-profile including to increased Y247, Y265, S279/282, S365, and S373 phosphorylation. Lysosomal inhibition, on the other hand, nearly eliminated phosphorylation on Y247 and Y265 and reduced S368 and S373 while increasing S279/282 phosphorylation levels. We present a model of gap junction disassembly where multiple modes of disassembly are regulated by phosphorylation and can have differential effects on cellular signaling. -

Minding the Calcium Store: Ryanodine Receptor Activation As a Convergent Mechanism of PCB Toxicity
Pharmacology & Therapeutics 125 (2010) 260–285 Contents lists available at ScienceDirect Pharmacology & Therapeutics journal homepage: www.elsevier.com/locate/pharmthera Associate Editor: Carey Pope Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity Isaac N. Pessah ⁎, Gennady Cherednichenko, Pamela J. Lein Department of Molecular Biosciences, School of Veterinary Medicine, University of California, Davis, CA 95616, USA article info abstract Keywords: Chronic low-level polychlorinated biphenyl (PCB) exposures remain a significant public health concern since Ryanodine receptor (RyR) results from epidemiological studies indicate that PCB burden is associated with immune system Calcium-induced calcium release dysfunction, cardiovascular disease, and impairment of the developing nervous system. Of these various Calcium regulation adverse health effects, developmental neurotoxicity has emerged as a particularly vulnerable endpoint in Polychlorinated biphenyls PCB toxicity. Arguably the most pervasive biological effects of PCBs could be mediated by their ability to alter Triclosan fi 2+ Bastadins the spatial and temporal delity of Ca signals through one or more receptor-mediated processes. This Polybrominated diphenylethers review will focus on our current knowledge of the structure and function of ryanodine receptors (RyRs) in Developmental neurotoxicity muscle and nerve cells and how PCBs and related non-coplanar structures alter these functions. The Activity dependent plasticity molecular and cellular mechanisms by which non-coplanar PCBs and related structures alter local and global Ca2+ signaling properties and the possible short and long-term consequences of these perturbations on neurodevelopment and neurodegeneration are reviewed. © 2009 Elsevier Inc. All rights reserved. Contents 1. Introduction ............................................... 260 2. Ryanodine receptor macromolecular complexes: significance to polychlorinated biphenyl-mediated Ca2+ dysregulation . -

Next Generation Sequencing for Molecular Confirmation of Hereditary
Arch Cardiol Mex. 2015;85(1):68---72 www.elsevier.com.mx SPECIAL ARTICLE Next generation sequencing for molecular confirmation of hereditary sudden cardiac death syndromes a,∗ b b b Manlio F. Márquez , David Cruz-Robles , Selene Ines-Real , Gilberto Vargas-Alarcón , a Manuel Cárdenas a Departamento de Electrofisiología, Instituto Nacional de Cardiología Ignacio Chávez, México, D.F., Mexico b Departamento de Biología Molecular, Instituto Nacional de Cardiología Ignacio Chávez, México, D.F., Mexico Received 26 March 2014; accepted 8 December 2014 KEYWORDS Abstract Hereditary sudden cardiac death syndromes comprise a wide range of diseases result- Arrhythmias; ing from alteration in cardiac ion channels. Genes involved in these syndromes represent diverse Hereditary sudden mutations that cause the altered encoding of the diverse proteins constituting these channels, cardiac death thus affecting directly the currents of the corresponding ions. In the present article we will syndromes; briefly review how to arrive to a clinical diagnosis and we will present the results of molecular Right ventricle genetic studies made in Mexican subjects attending the SCD Syndromes Clinic of the National arrhythmogenic Institute of Cardiology of Mexico City. cardiomyopathy; © 2014 Instituto Nacional de Cardiología Ignacio Chávez. Published by Masson Doyma México Brugada syndrome S.A. All rights reserved. PALABRAS CLAVE Confirmación diagnóstica molecular mediante secuenciación masiva de nueva Arritmias; generación (‘‘next generation sequencing’’) en síndromes hereditarios de muerte Síndromes súbita cardíaca hereditarios de Resumen Los síndromes hereditarios de muerte súbita cardíaca comprenden una amplia gama muerte súbita; Displasia de enfermedades resultantes de la alteración en los canales iónicos cardíacos. Los genes implicados en estos síndromes presentan mutaciones que causan alteraciones de las diversas Arritmogénica del proteínas que constituyen estos canales y que, por lo tanto, afectan directamente a las difer- ventriculo derecho; entes corrientes iónicas. -

Non-Coding Rnas in the Cardiac Action Potential and Their Impact on Arrhythmogenic Cardiac Diseases
Review Non-Coding RNAs in the Cardiac Action Potential and Their Impact on Arrhythmogenic Cardiac Diseases Estefania Lozano-Velasco 1,2 , Amelia Aranega 1,2 and Diego Franco 1,2,* 1 Cardiovascular Development Group, Department of Experimental Biology, University of Jaén, 23071 Jaén, Spain; [email protected] (E.L.-V.); [email protected] (A.A.) 2 Fundación Medina, 18016 Granada, Spain * Correspondence: [email protected] Abstract: Cardiac arrhythmias are prevalent among humans across all age ranges, affecting millions of people worldwide. While cardiac arrhythmias vary widely in their clinical presentation, they possess shared complex electrophysiologic properties at cellular level that have not been fully studied. Over the last decade, our current understanding of the functional roles of non-coding RNAs have progressively increased. microRNAs represent the most studied type of small ncRNAs and it has been demonstrated that miRNAs play essential roles in multiple biological contexts, including normal development and diseases. In this review, we provide a comprehensive analysis of the functional contribution of non-coding RNAs, primarily microRNAs, to the normal configuration of the cardiac action potential, as well as their association to distinct types of arrhythmogenic cardiac diseases. Keywords: cardiac arrhythmia; microRNAs; lncRNAs; cardiac action potential Citation: Lozano-Velasco, E.; Aranega, A.; Franco, D. Non-Coding RNAs in the Cardiac Action Potential 1. The Electrical Components of the Adult Heart and Their Impact on Arrhythmogenic The adult heart is a four-chambered organ that propels oxygenated blood to the entire Cardiac Diseases. Hearts 2021, 2, body. It is composed of atrial and ventricular chambers, each of them with distinct left and 307–330. -

Development of the Stria Vascularis and Potassium Regulation in the Human Fetal Cochlea: Insights Into Hereditary Sensorineural Hearing Loss
Development of the Stria Vascularis and Potassium Regulation in the Human Fetal Cochlea: Insights into Hereditary Sensorineural Hearing Loss Heiko Locher,1,2 John C.M.J. de Groot,2 Liesbeth van Iperen,1 Margriet A. Huisman,2 Johan H.M. Frijns,2 Susana M. Chuva de Sousa Lopes1,3 1 Department of Anatomy and Embryology, Leiden University Medical Center, Leiden, 2333 ZA, the Netherlands 2 Department of Otorhinolaryngology and Head and Neck Surgery, Leiden University Medical Center, Leiden, 2333 ZA, the Netherlands 3 Department for Reproductive Medicine, Ghent University Hospital, 9000 Ghent, Belgium Received 25 August 2014; revised 2 February 2015; accepted 2 February 2015 ABSTRACT: Sensorineural hearing loss (SNHL) is dynamics of key potassium-regulating proteins. At W12, one of the most common congenital disorders in humans, MITF1/SOX101/KIT1 neural-crest-derived melano- afflicting one in every thousand newborns. The majority cytes migrated into the cochlea and penetrated the base- is of heritable origin and can be divided in syndromic ment membrane of the lateral wall epithelium, and nonsyndromic forms. Knowledge of the expression developing into the intermediate cells of the stria vascula- profile of affected genes in the human fetal cochlea is lim- ris. These melanocytes tightly integrated with Na1/K1- ited, and as many of the gene mutations causing SNHL ATPase-positive marginal cells, which started to express likely affect the stria vascularis or cochlear potassium KCNQ1 in their apical membrane at W16. At W18, homeostasis (both essential to hearing), a better insight KCNJ10 and gap junction proteins GJB2/CX26 and into the embryological development of this organ is GJB6/CX30 were expressed in the cells in the outer sul- needed to understand SNHL etiologies. -
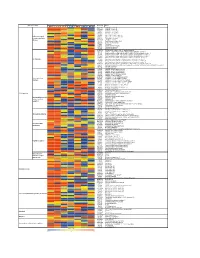
Supporting Figure 2
Normalized value Functional class Genbank Name Naive SC 1h SC 6h SC 24h WM 1h WM 6h WM 24h AB016161 GABA-B receptor 1d AF109405 GABA-B receptor 2a M35077 Dopamine-1A receptor S46131 Dopamine-1A receptor M84009 Dopamine receptor D4 U13368 Adrenergic receptor, alpha 1a G Protein-coupled M60654 Adrenergic receptor, alpha 1d receptors and their M64236 Tachykinin 1 receptor effectors AI229237 Opioid receptor-like Y11433 Pyrimidinergic receptor P2Y4 M64299 Adenosine A1 receptor E00001 Pro-insulin M29014 Insulin receptor precursor U35315 Serotonin receptor 2C S62043 Serotonin receptor 6 AF000368 Scn9a sodium channel, type IX, alpha polypeptide M27158 Kcna5 K+ voltage-gated channel, shaker-related subfamily, member 5 X17621 Kcna6 potassium voltage-gated channel, shaker-related, subfamily, member 6 X16476 Kcnb1 potassium voltage gated channel, Shab-related subfamily, member 1 M77482 Kcnb2 potassium voltage gated channel, Shab-related subfamily, member 2 S64320 Kcnd2 potassium voltage gated channel, Shal-related family, member 2 Ion channels X87635 Kcnj4 potassium inwardly-rectifying channel, subfamily J, member 4 D86039 Kcnj11 potassium inwardly-rectifying channel, subfamily J, member 11 X83581 Kcnj16 potassium inwardly-rectifying channel, subfamily J, member 16 AF073891 Kcnh5 potassium voltage-gated channel, subfamily H (eag-related), member 5 U69882 Kcnn2 potassium intermediate/small conductance calcium-activated channel, subfamily N, member 2 Z36944 Chloride channel 4-2 Z56277 Chloride channel 5 L08493 GABA-A receptor alpha-4 subunit X51992 -

Atrial Fibrillation (ATRIA) Study
European Journal of Human Genetics (2014) 22, 297–306 & 2014 Macmillan Publishers Limited All rights reserved 1018-4813/14 www.nature.com/ejhg REVIEW Atrial fibrillation: the role of common and rare genetic variants Morten S Olesen*,1,2,4, Morten W Nielsen1,2,4, Stig Haunsø1,2,3 and Jesper H Svendsen1,2,3 Atrial fibrillation (AF) is the most common cardiac arrhythmia affecting 1–2% of the general population. A number of studies have demonstrated that AF, and in particular lone AF, has a substantial genetic component. Monogenic mutations in lone and familial AF, although rare, have been recognized for many years. Presently, mutations in 25 genes have been associated with AF. However, the complexity of monogenic AF is illustrated by the recent finding that both gain- and loss-of-function mutations in the same gene can cause AF. Genome-wide association studies (GWAS) have indicated that common single-nucleotide polymorphisms (SNPs) have a role in the development of AF. Following the first GWAS discovering the association between PITX2 and AF, several new GWAS reports have identified SNPs associated with susceptibility of AF. To date, nine SNPs have been associated with AF. The exact biological pathways involving these SNPs and the development of AF are now starting to be elucidated. Since the first GWAS, the number of papers concerning the genetic basis of AF has increased drastically and the majority of these papers are for the first time included in a review. In this review, we discuss the genetic basis of AF and the role of both common and rare genetic variants in the susceptibility of developing AF. -

Engineering Biosynthetic Excitable Tissues from Unexcitable Cells for Electrophysiological and Cell Therapy Studies
ARTICLE Received 11 Nov 2010 | Accepted 5 Apr 2011 | Published 10 May 2011 DOI: 10.1038/ncomms1302 Engineering biosynthetic excitable tissues from unexcitable cells for electrophysiological and cell therapy studies Robert D. Kirkton1 & Nenad Bursac1 Patch-clamp recordings in single-cell expression systems have been traditionally used to study the function of ion channels. However, this experimental setting does not enable assessment of tissue-level function such as action potential (AP) conduction. Here we introduce a biosynthetic system that permits studies of both channel activity in single cells and electrical conduction in multicellular networks. We convert unexcitable somatic cells into an autonomous source of electrically excitable and conducting cells by stably expressing only three membrane channels. The specific roles that these expressed channels have on AP shape and conduction are revealed by different pharmacological and pacing protocols. Furthermore, we demonstrate that biosynthetic excitable cells and tissues can repair large conduction defects within primary 2- and 3-dimensional cardiac cell cultures. This approach enables novel studies of ion channel function in a reproducible tissue-level setting and may stimulate the development of new cell-based therapies for excitable tissue repair. 1 Department of Biomedical Engineering, Duke University, Durham, North Carolina 27708, USA. Correspondence and requests for materials should be addressed to N.B. (email: [email protected]). NatURE COMMUNicatiONS | 2:300 | DOI: 10.1038/ncomms1302 | www.nature.com/naturecommunications © 2011 Macmillan Publishers Limited. All rights reserved. ARTICLE NatUre cOMMUNicatiONS | DOI: 10.1038/ncomms1302 ll cells express ion channels in their membranes, but cells a b with a significantly polarized membrane that can undergo e 0 a transient all-or-none membrane depolarization (action A 1 potential, AP) are classified as ‘excitable cells’ . -

Ion Channels 3 1
r r r Cell Signalling Biology Michael J. Berridge Module 3 Ion Channels 3 1 Module 3 Ion Channels Synopsis Ion channels have two main signalling functions: either they can generate second messengers or they can function as effectors by responding to such messengers. Their role in signal generation is mainly centred on the Ca2 + signalling pathway, which has a large number of Ca2+ entry channels and internal Ca2+ release channels, both of which contribute to the generation of Ca2 + signals. Ion channels are also important effectors in that they mediate the action of different intracellular signalling pathways. There are a large number of K+ channels and many of these function in different + aspects of cell signalling. The voltage-dependent K (KV) channels regulate membrane potential and + excitability. The inward rectifier K (Kir) channel family has a number of important groups of channels + + such as the G protein-gated inward rectifier K (GIRK) channels and the ATP-sensitive K (KATP) + + channels. The two-pore domain K (K2P) channels are responsible for the large background K current. Some of the actions of Ca2 + are carried out by Ca2+-sensitive K+ channels and Ca2+-sensitive Cl − channels. The latter are members of a large group of chloride channels and transporters with multiple functions. There is a large family of ATP-binding cassette (ABC) transporters some of which have a signalling role in that they extrude signalling components from the cell. One of the ABC transporters is the cystic − − fibrosis transmembrane conductance regulator (CFTR) that conducts anions (Cl and HCO3 )and contributes to the osmotic gradient for the parallel flow of water in various transporting epithelia.