Cortical Ischaemic Patterns in Term Partial-Prolonged Hypoxic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endovascular Treatment of Stroke Caused by Carotid Artery Dissection
brain sciences Case Report Endovascular Treatment of Stroke Caused by Carotid Artery Dissection Grzegorz Meder 1,* , Milena Swito´ ´nska 2,3 , Piotr Płeszka 2, Violetta Palacz-Duda 2, Dorota Dzianott-Pabijan 4 and Paweł Sokal 3 1 Department of Interventional Radiology, Jan Biziel University Hospital No. 2, Ujejskiego 75 Street, 85-168 Bydgoszcz, Poland 2 Stroke Intervention Centre, Department of Neurosurgery and Neurology, Jan Biziel University Hospital No. 2, Ujejskiego 75 Street, 85-168 Bydgoszcz, Poland; [email protected] (M.S.);´ [email protected] (P.P.); [email protected] (V.P.-D.) 3 Department of Neurosurgery and Neurology, Faculty of Health Sciences, Nicolaus Copernicus University in Toru´n,Ludwik Rydygier Collegium Medicum, Ujejskiego 75 Street, 85-168 Bydgoszcz, Poland; [email protected] 4 Neurological Rehabilitation Ward Kuyavian-Pomeranian Pulmonology Centre, Meysnera 9 Street, 85-472 Bydgoszcz, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-52-3655-143; Fax: +48-52-3655-364 Received: 23 September 2020; Accepted: 27 October 2020; Published: 30 October 2020 Abstract: Ischemic stroke due to large vessel occlusion (LVO) is a devastating condition. Most LVOs are embolic in nature. Arterial dissection is responsible for only a small proportion of LVOs, is specific in nature and poses some challenges in treatment. We describe 3 cases where patients with stroke caused by carotid artery dissection were treated with mechanical thrombectomy and extensive stenting with good outcome. We believe that mechanical thrombectomy and stenting is a treatment of choice in these cases. Keywords: stroke; artery dissection; endovascular treatment; stenting; mechanical thrombectomy 1. -

Download PDF File
ONLINE FIRST This is a provisional PDF only. Copyedited and fully formatted version will be made available soon. ISSN: 0015-5659 e-ISSN: 1644-3284 Two cases of combined anatomical variations: maxillofacial trunk, vertebral, posterior communicating and anterior cerebral atresia, linguofacial and labiomental trunks Authors: M. C. Rusu, A. M. Jianu, M. D. Monea, A. C. Ilie DOI: 10.5603/FM.a2021.0007 Article type: Case report Submitted: 2020-11-28 Accepted: 2021-01-08 Published online: 2021-01-29 This article has been peer reviewed and published immediately upon acceptance. It is an open access article, which means that it can be downloaded, printed, and distributed freely, provided the work is properly cited. Articles in "Folia Morphologica" are listed in PubMed. Powered by TCPDF (www.tcpdf.org) Two cases of combined anatomical variations: maxillofacial trunk, vertebral, posterior communicating and anterior cerebral atresia, linguofacial and labiomental trunks M.C. Rusu et al., The maxillofacial trunk M.C. Rusu1, A.M. Jianu2, M.D. Monea2, A.C. Ilie3 1Division of Anatomy, Faculty of Dental Medicine, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania 2Department of Anatomy, Faculty of Medicine, “Victor Babeş” University of Medicine and Pharmacy, Timişoara, Romania 3Department of Functional Sciences, Discipline of Public Health, Faculty of Medicine, “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania Address for correspondence: M.C. Rusu, MD, PhD (Med.), PhD (Biol.), Dr. Hab., Prof., Division of Anatomy, Faculty of Dental Medicine, “Carol Davila” University of Medicine and Pharmacy, 8 Eroilor Sanitari Blvd., RO-76241, Bucharest, Romania, , tel: +40722363705 e-mail: [email protected] ABSTRACT Background: Commonly, arterial anatomic variants are reported as single entities. -

Mechanical Thrombectomy in Basilar Artery Occlusion Presence of Bilateral Posterior Communicating Arteries Is a Predictor of Favorable Clinical Outcome
Clin Neuroradiol (2019) 29:153–160 https://doi.org/10.1007/s00062-017-0651-3 ORIGINAL ARTICLE Mechanical Thrombectomy in Basilar Artery Occlusion Presence of Bilateral Posterior Communicating Arteries is a Predictor of Favorable Clinical Outcome Volker Maus 1 ·AlevKalkan1 · Christoph Kabbasch1 · Nuran Abdullayev1 · Henning Stetefeld2 · Utako Birgit Barnikol3 · Thomas Liebig4 · Christian Dohmen2 · Gereon Rudolf Fink2,5 · Jan Borggrefe1 · Anastasios Mpotsaris6 Received: 17 August 2017 / Accepted: 21 November 2017 / Published online: 19 December 2017 © Springer-Verlag GmbH Germany, part of Springer Nature 2017 Abstract Results The favorable clinical outcome at 90 days was 25% Background Mechanical thrombectomy (MT) of basilar and mortality was 43%. The rate of successful reperfusion, artery occlusions (BAO) is a subject of debate. We inves- i.e. modified thrombolysis in cerebral infarction (mTICI) ≥ tigated the clinical outcome of MT in BAO and predictors 2b was 82%. Presence of bilateral PcoAs (area under the of a favorable outcome. curve, AUC: 0.81, odds ratio, OR: 4.2, 2.2–8.2; p < 0.0001), Material and Methods A total of 104 MTs of BAO (carried lower National Institute of Health Stroke Scale (NIHSS) out between 2010 and 2016) were analyzed. Favorable out- on admission (AUC: 0.74, OR: 2.6, 1.3–5.2; p < 0.01), PC- come as a modified Rankin scale (mRS) Ä 2at90days ASPECTS ≥ 9 (AUC: 0.72, OR: 4.2, 1.5–11.9; p < 0.01), was the primary endpoint. The influence of the follow- incomplete BAO (AUC: 0.66, OR: 2.6, 1.4–4.8; p < 0.001), ing variables on outcome was investigated: number of de- and basilar tip patency (AUC: 0.66, OR: 2.5, 1.3–4.8; p < tectable posterior communicating arteries (PcoAs), patency 0.01) were associated with a favorable outcome. -

Imaging Surgical Epilepsy in Children
Childs Nerv Syst (2006) 22:786–809 DOI 10.1007/s00381-006-0132-5 SPECIAL ANNUAL ISSUE Imaging surgical epilepsy in children Charles Raybaud & Manohar Shroff & James T. Rutka & Sylvester H. Chuang Received: 1 February 2006 / Published online: 13 June 2006 # Springer-Verlag 2006 Abstract of the diverse pathologies concerned with epilepsy surgery in Introduction Epilepsy surgery rests heavily upon magnetic the pediatric context is provided with illustrative images. resonance imaging (MRI). Technical developments have brought significantly improved efficacy of MR imaging in Keywords MR imaging . Epilepsy. Ganglioglioma . DNT. detecting and assessing surgical epileptogenic lesions, PXA . FCD . Hypothalamic hamartoma . while more clinical experience has brought better definition Meningioangiomatosis of the pathological groups. Discussion MRI is fairly efficient in identifying develop- mental, epilepsy-associated tumors such as ganglioglioma Introduction (with its variants gangliocytoma and desmoplastic infantile ganglioglioma), the complex, simple and nonspecific forms Epilepsy surgery in children addresses severe refractory of dysembryoplastic neuroepithelial tumor, and the rare epilepsy mainly, which threatens the child’s development, pleomorphic xanthoastrocytoma. The efficacy of MR when a constant epileptogenic focus can be identified from imaging is not as good for the diagnosis of focal cortical the clinical and the electroencephalogram (EEG) data and be dysplasia (FCD), as it does not necessarily correlate with removed. Imaging must demonstrate -
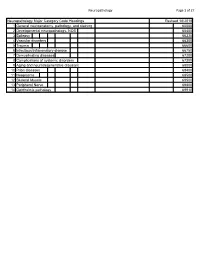
Neuropathology Category Code List
Neuropathology Page 1 of 27 Neuropathology Major Category Code Headings Revised 10/2018 1 General neuroanatomy, pathology, and staining 65000 2 Developmental neuropathology, NOS 65400 3 Epilepsy 66230 4 Vascular disorders 66300 5 Trauma 66600 6 Infectious/inflammatory disease 66750 7 Demyelinating diseases 67200 8 Complications of systemic disorders 67300 9 Aging and neurodegenerative diseases 68000 10 Prion diseases 68400 11 Neoplasms 68500 12 Skeletal Muscle 69500 13 Peripheral Nerve 69800 14 Ophthalmic pathology 69910 Neuropathology Page 2 of 27 Neuropathology 1 General neuroanatomy, pathology, and staining 65000 A Neuroanatomy, NOS 65010 1 Neocortex 65011 2 White matter 65012 3 Entorhinal cortex/hippocampus 65013 4 Deep (basal) nuclei 65014 5 Brain stem 65015 6 Cerebellum 65016 7 Spinal cord 65017 8 Pituitary 65018 9 Pineal 65019 10 Tracts 65020 11 Vascular supply 65021 12 Notochord 65022 B Cell types 65030 1 Neurons 65031 2 Astrocytes 65032 3 Oligodendroglia 65033 4 Ependyma 65034 5 Microglia and mononuclear cells 65035 6 Choroid plexus 65036 7 Meninges 65037 8 Blood vessels 65038 C Cerebrospinal fluid 65045 D Pathologic responses in neurons and axons 65050 1 Axonal degeneration/spheroid/reaction 65051 2 Central chromatolysis 65052 3 Tract degeneration 65053 4 Swollen/ballooned neurons 65054 5 Trans-synaptic neuronal degeneration 65055 6 Olivary hypertrophy 65056 7 Acute ischemic (hypoxic) cell change 65057 8 Apoptosis 65058 9 Protein aggregation 65059 10 Protein degradation/ubiquitin pathway 65060 E Neuronal nuclear inclusions 65100 -

Diffusion Weighted Imaging in Pediatric Neuroradiology Massachusetts General Hospital, Boston, MA • Harvard Medical School, Boston, MA
Pallavi Sagar, M.D.; P. Ellen Grant, M.D. Diffusion Weighted Imaging in Pediatric Neuroradiology Massachusetts General Hospital, Boston, MA • Harvard Medical School, Boston, MA I. CEREBROVASCULAR DISEASE IV. TOXIC AND METABOLIC DISORDERS V. DEMYELINATING DISORDERS 1) Arterial Ischemic Stroke (AIS) 3) Global Hypoxia and Hypoperfusion 1) Methotrexate Neurotoxicity 1) Multiple Sclerosis (MS) INTRODUCTION Pediatric AIS is less common and almost 50% of the pediatric AIS are Perinatal Hypoxic Ischemic Brain Injury Intrathecal or intravenous methotrexate can cross blood brain barrier and cause diffuse or multifocal Figure 9. Methotrexate toxicity. Axial (a) T2 and (b) DWI. There is MS is an inflammatory demyelinating process, Diffusion-weighted magnetic resonance idiopathic. Pediatric strokes can be classified as perinatal (between 28 weeks white matter changes typically in the periventricular region. Alteration in myelin metabolism faint T2 hyperintensity in the left posterior centrum semiovale. which can be reversible due to reparative imaging (DWI) provides image contrast DWI is more sensitive in demonstrating increased signal in this of gestation and 28 days of postnatal life) or childhood (between 30 days and Hypoxic ischemic brain injury results from a combination of global cerebral hypoxia and hypoperfusion. Two patterns have causing axonal swelling and intramyelinic edema has been proposed. In acute encephalopathy region and an additional lesion on the right side presumably remyelination or become irreversible with that is dependent on the molecular motion 18 year of life) ( Figure 1) . been described. Partial asphyxia or peripheral pattern with bilateral white matter injury and a profound or central pattern with transient and reversible lesions with decreased diffusion can be observed. -

Os Odontoideum As a Cause of Cervical Cord Injury in a Patient with Refractory Epilepsy
Case Report Clinics in Surgery Published: 09 Feb, 2021 Os Odontoideum as a Cause of Cervical Cord Injury in a Patient with Refractory Epilepsy Shohei Kusabiraki1, Eiji Nakagawa1*, Takashi Saito1, Yutaro Takayama2, Keiya Iijima2, Masaki Iwasaki2, Ayano Matsui3 and Tetsuya Abe4 1Department of Child Neurology, National Center Hospital, NCNP, Japan 2Department of Neurosurgery, National Center Hospital, Japan 3Department of Orthopedics, National Center Hospital, Japan 4Department of Orthopedic Surgery, University of Tsukuba, Japan Abstract Os odontoideum is an anomaly of the second cervical vertebrae in which the odontoid process is separated from the body of the axis. Traumatic injury or congenital fusion failure is thought to be the etiology. The clinical symptoms are variable from cervical pain, torticollis, and myelopathy and vertebrobasilar ischemia. Os odontoideum can cause instability of the neck, and neck injuries can cause life-threatening complications. In this report, we present the case of a 15-year-old girl with refractory epilepsy who developed quadriparesis after a fall and hit to the forehead while traveling. Although the symptoms improved, weakness in her right upper limb persisted at 2 months after the fall. Imaging studies revealed Os odontoideum. Based on her medical history, the recent head trauma due to epileptic seizures accompanied by atlantoaxial instability was considered to result in cervical compression and spinal damage. She was at a high risk of sudden death due to recurrent seizures and cervical injury; therefore, -

UC San Francisco Previously Published Works
UCSF UC San Francisco Previously Published Works Title A pictorial review of the pathophysiology and classification of the magnetic resonance imaging patterns of perinatal term hypoxic ischemic brain injury - What the radiologist needs to know…. Permalink https://escholarship.org/uc/item/0s5968ds Journal SA journal of radiology, 24(1) ISSN 1027-202X Authors Misser, Shalendra K Barkovich, Anthony J Lotz, Jan W et al. Publication Date 2020 DOI 10.4102/sajr.v24i1.1915 Peer reviewed eScholarship.org Powered by the California Digital Library University of California SA Journal of Radiology ISSN: (Online) 2078-6778, (Print) 1027-202X Page 1 of 17 Pictorial Review A pictorial review of the pathophysiology and classification of the magnetic resonance imaging patterns of perinatal term hypoxic ischemic brain injury – What the radiologist needs to know… Authors: This article provides a correlation of the pathophysiology and magnetic resonance imaging 1,2 Shalendra K. Misser (MRI) patterns identified on imaging of children with hypoxic ischemic brain injury (HIBI). Anthony J. Barkovich3 Jan W. Lotz4 The purpose of this pictorial review is to empower the reading radiologist with a simplified Moherndran Archary5 classification of the patterns of cerebral injury matched to images of patients demonstrating each subtype. A background narrative literature review was undertaken of the regional, Affiliations: continental and international databases looking at specific patterns of cerebral injury related to 1Department of Radiology, Faculty of Health Sciences perinatal HIBI. In addition, a database of MRI studies accumulated over a decade (including a Medicine, College of Health total of 314 studies) was analysed and subclassified into the various patterns of cerebral injury. -
THE SYNDROMES of the ARTERIES of the BRAIN AND, SPINAL CORD Part II by LESLIE G
I19 Postgrad Med J: first published as 10.1136/pgmj.29.329.119 on 1 March 1953. Downloaded from - N/ THE SYNDROMES OF THE ARTERIES OF THE BRAIN AND, SPINAL CORD Part II By LESLIE G. KILOH, M.D., M.R.C.P., D.P.M. First Assistant in the Joint Department of Psychological Medicine, Royal Victoria Infirmary and University of Durham The Vertebral Artery (See also Cabot, I937; Pines and Gilensky, Each vertebral artery enters the foramen 1930.) magnum in front of the roots of the hypoglossal nerve, inclines forwards and medially to the The Posterior Inferior Cerebellar Artery anterior aspect of the medulla oblongata and unites The posterior inferior cerebellar artery arises with its fellow at the lower border of the pons to from the vertebral artery at the level of the lower form the basilar artery. border of the inferior olive and winds round the The posterior inferior cerebellar and the medulla oblongata between the roots of the hypo- Protected by copyright. anterior spinal arteries are its principal branches glossal nerve. It passes rostrally behind the root- and it sometimes gives off the posterior spinal lets of the vagus and glossopharyngeal nerves to artery. A few small branches are supplied directly the lower border of the pons, bends backwards and to the medulla oblongata. These are in line below caudally along the inferolateral boundary of the with similar branches of the anterior spinal artery fourth ventricle and finally turns laterally into the and above with the paramedian branches of the vallecula. basilar artery. Branches: From the trunk of the artery, In some cases of apparently typical throm- twigs enter the lateral aspect of the medulla bosis of the posterior inferior cerebellar artery, oblongata and supply the region bounded ventrally post-mortem examination has demonstrated oc- by the inferior olive and medially by the hypo- clusion of the entire vertebral artery (e.g., Diggle glossal nucleus-including the nucleus ambiguus, and Stcpford, 1935). -

Vascularization of the Alouatta Belzebul Brain Base1 Dayane Kelly Sabec-Pereira2*, Fabiano C
Pesq. Vet. Bras. 40(4):315-323, April 2020 DOI: 10.1590/1678-5150-PVB-6536 Original Article Animal Morphophysiology ISSN 0100-736X (Print) ISSN 1678-5150 (Online) PVB-6536 MF Vascularization of the Alouatta belzebul brain base1 Dayane Kelly Sabec-Pereira2*, Fabiano C. Lima3, Fabiano R. Melo4, Fabiana Cristina S.A. Melo4, Kleber Fernando Pereira5 and Valcinir Aloisio S. Vulcani2.3 ABSTRACT.- Sabec-Pereira D.K., Lima F.C., Melo F.R., Melo F.C.S.A., Pereira K.F. & Vulcani V.A.S. 2020. Vascularization of the Alouatta belzebul brain base. Pesquisa Veterinária Brasileira 40(4):315-323. Escola de Veterinária e Zootecnia, Universidade Federal de Goiás, Avenida Esperança s/n, Campus Samambaia, Goiânia, GO 74690-900, Brazil. E-mail: [email protected] Alouatta belzebul primate. The material had the arterial system perfused (water at 40°C), injected with stained We studied the arterial circle in the brain of five specimens of the Título Original latexthe vertebra-basilar (Neoprene 650), and fixed the carotidin aqueous ones, formaldehyde which anastomose solution to close (10%) the andarterial dissected circuit. for In vesselthe caudal verification. portion ofThe the arterial arterial circle circle, of therethis primate are the isvertebral composed arteries of two and vascular their branches: systems: the rostral spinal artery and the caudal inferior cerebellar artery. The anastomosis of the [Título traduzido]. vertebral arteries gives rise to the basilar artery. It presented an anatomical variation at the beginning of its path, forming a double basilar artery, called arterial island. In its course, it emitted branches giving rise to the rostral inferior cerebellar artery, the pontine arteries, the rostral cerebellar arteries, the satellite rostral cerebellar arteries and its terminal branch, Autores the caudal cerebral artery, which presented itself in two segments: the pre-communicating one and post-communicating, joining the internal carotid artery and originating the caudal communicating artery. -

Study of Variant Posterior Cerebral Circulation and Its Clinical Relevance
ogy: iol Cu ys r h re P n t & R y e s Anatomy & Physiology: Current m e o a t r a c n h Sawanth and Rizvi, Anat Physiol 2017, 7:3 A Research ISSN: 2161-0940 DOI: 10.4172/2161-0940.1000264 Research Article Open Access Study of Variant Posterior Cerebral Circulation and its Clinical Relevance Sawant SP* and Rizvi S Department of Anatomy, KJ Somaiya Medical College, Somaiya Ayurvihar, Eastern Express Highway, Sion, Mumbai, India *Corresponding author: Sawanth SP, Department of Anatomy, KJ Somaiya Medical College, Somaiya Ayurvihar, Eastern Express Highway, Sion, Mumbai, India, Tel: +9322061220; E-mail: [email protected] Received Date: April 24, 2017; Accepted Date: May 03, 2017; Published Date: May 10, 2017 Copyright: © 2017 Sawanth SP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract The cerebral blood flow is divided into an anterior circulation and a posterior circulation connected to each other in the form of a circle called Circle of Willis (CW). It is formed by the unification of the internal carotid (ICA) and vertebrobasilar systems Posteriorly, the basilar artery, formed by the left and right vertebral arteries, branches into a left and right posterior cerebral artery (PCA), forming the posterior circulation. The internal carotid system lies anteriorly and is joined to the posterior circulation by posterior communicating (PCoA) arteries. The internal carotid artery divides into anterior and middle cerebral artery. -

The Role of Inhibitory Interneurons in a Model Od Developmental Epilepsy
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2007 The Role of Inhibitory Interneurons in a Model od Developmental Epilepsy Patrick James Wolfgang Virginia Commonwealth University Follow this and additional works at: https://scholarscompass.vcu.edu/etd Part of the Nervous System Commons © The Author Downloaded from https://scholarscompass.vcu.edu/etd/1145 This Thesis is brought to you for free and open access by the Graduate School at VCU Scholars Compass. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. © Patrick James Wolfgang 2007 All Rights Reserved THE ROLE OF INHIBITORY INTERNEURONS IN A MODEL OF DEVELOPMENTAL EPILEPSY A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Anatomy and Neurobiology at Virginia Commonwealth University. by PATRICK JAMES WOLFGANG BA Environmental Science, University of Virginia, 2005 Director: KIMBERLE JACOBS ASSISTANT PROFESSOR ANATOMY AND NEUROBIOLOGY Virginia Commonwealth University Richmond, Virginia August, 2007 ii Acknowledgement First, I would like to thank my parents for their support over the last 25 years. I would also like to thank Dr. Jacobs for her support and guidance during the year. I would also like to extend a sincere thanks to Amanda Lynn George (AKA Mandy 5 or the MD/PhDbot) for keeping me sane as the year progressed. I expect to see your name quite a bit in future publications. I would also like to thank everyone else who has supported me over the years: Michael, Katie, Matthew, Matt, Rob, Hatcher, Tommy, The other Tommy, Patrick, Lauren, Teo, Cara, Shep, Alex, Jamie, Malcolm, Kelly, Shep, Moo, Markowitz, Matt, Jordan, Tina, Kim, Anya, Melissa, Lynn, Sue, all of my teachers, Bam Bam, Grace, the whole Mendez family, the Longos, the Estes, Ms.