UC San Francisco Previously Published Works
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Imaging Surgical Epilepsy in Children
Childs Nerv Syst (2006) 22:786–809 DOI 10.1007/s00381-006-0132-5 SPECIAL ANNUAL ISSUE Imaging surgical epilepsy in children Charles Raybaud & Manohar Shroff & James T. Rutka & Sylvester H. Chuang Received: 1 February 2006 / Published online: 13 June 2006 # Springer-Verlag 2006 Abstract of the diverse pathologies concerned with epilepsy surgery in Introduction Epilepsy surgery rests heavily upon magnetic the pediatric context is provided with illustrative images. resonance imaging (MRI). Technical developments have brought significantly improved efficacy of MR imaging in Keywords MR imaging . Epilepsy. Ganglioglioma . DNT. detecting and assessing surgical epileptogenic lesions, PXA . FCD . Hypothalamic hamartoma . while more clinical experience has brought better definition Meningioangiomatosis of the pathological groups. Discussion MRI is fairly efficient in identifying develop- mental, epilepsy-associated tumors such as ganglioglioma Introduction (with its variants gangliocytoma and desmoplastic infantile ganglioglioma), the complex, simple and nonspecific forms Epilepsy surgery in children addresses severe refractory of dysembryoplastic neuroepithelial tumor, and the rare epilepsy mainly, which threatens the child’s development, pleomorphic xanthoastrocytoma. The efficacy of MR when a constant epileptogenic focus can be identified from imaging is not as good for the diagnosis of focal cortical the clinical and the electroencephalogram (EEG) data and be dysplasia (FCD), as it does not necessarily correlate with removed. Imaging must demonstrate -
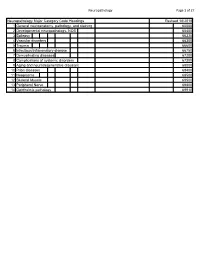
Neuropathology Category Code List
Neuropathology Page 1 of 27 Neuropathology Major Category Code Headings Revised 10/2018 1 General neuroanatomy, pathology, and staining 65000 2 Developmental neuropathology, NOS 65400 3 Epilepsy 66230 4 Vascular disorders 66300 5 Trauma 66600 6 Infectious/inflammatory disease 66750 7 Demyelinating diseases 67200 8 Complications of systemic disorders 67300 9 Aging and neurodegenerative diseases 68000 10 Prion diseases 68400 11 Neoplasms 68500 12 Skeletal Muscle 69500 13 Peripheral Nerve 69800 14 Ophthalmic pathology 69910 Neuropathology Page 2 of 27 Neuropathology 1 General neuroanatomy, pathology, and staining 65000 A Neuroanatomy, NOS 65010 1 Neocortex 65011 2 White matter 65012 3 Entorhinal cortex/hippocampus 65013 4 Deep (basal) nuclei 65014 5 Brain stem 65015 6 Cerebellum 65016 7 Spinal cord 65017 8 Pituitary 65018 9 Pineal 65019 10 Tracts 65020 11 Vascular supply 65021 12 Notochord 65022 B Cell types 65030 1 Neurons 65031 2 Astrocytes 65032 3 Oligodendroglia 65033 4 Ependyma 65034 5 Microglia and mononuclear cells 65035 6 Choroid plexus 65036 7 Meninges 65037 8 Blood vessels 65038 C Cerebrospinal fluid 65045 D Pathologic responses in neurons and axons 65050 1 Axonal degeneration/spheroid/reaction 65051 2 Central chromatolysis 65052 3 Tract degeneration 65053 4 Swollen/ballooned neurons 65054 5 Trans-synaptic neuronal degeneration 65055 6 Olivary hypertrophy 65056 7 Acute ischemic (hypoxic) cell change 65057 8 Apoptosis 65058 9 Protein aggregation 65059 10 Protein degradation/ubiquitin pathway 65060 E Neuronal nuclear inclusions 65100 -

Diffusion Weighted Imaging in Pediatric Neuroradiology Massachusetts General Hospital, Boston, MA • Harvard Medical School, Boston, MA
Pallavi Sagar, M.D.; P. Ellen Grant, M.D. Diffusion Weighted Imaging in Pediatric Neuroradiology Massachusetts General Hospital, Boston, MA • Harvard Medical School, Boston, MA I. CEREBROVASCULAR DISEASE IV. TOXIC AND METABOLIC DISORDERS V. DEMYELINATING DISORDERS 1) Arterial Ischemic Stroke (AIS) 3) Global Hypoxia and Hypoperfusion 1) Methotrexate Neurotoxicity 1) Multiple Sclerosis (MS) INTRODUCTION Pediatric AIS is less common and almost 50% of the pediatric AIS are Perinatal Hypoxic Ischemic Brain Injury Intrathecal or intravenous methotrexate can cross blood brain barrier and cause diffuse or multifocal Figure 9. Methotrexate toxicity. Axial (a) T2 and (b) DWI. There is MS is an inflammatory demyelinating process, Diffusion-weighted magnetic resonance idiopathic. Pediatric strokes can be classified as perinatal (between 28 weeks white matter changes typically in the periventricular region. Alteration in myelin metabolism faint T2 hyperintensity in the left posterior centrum semiovale. which can be reversible due to reparative imaging (DWI) provides image contrast DWI is more sensitive in demonstrating increased signal in this of gestation and 28 days of postnatal life) or childhood (between 30 days and Hypoxic ischemic brain injury results from a combination of global cerebral hypoxia and hypoperfusion. Two patterns have causing axonal swelling and intramyelinic edema has been proposed. In acute encephalopathy region and an additional lesion on the right side presumably remyelination or become irreversible with that is dependent on the molecular motion 18 year of life) ( Figure 1) . been described. Partial asphyxia or peripheral pattern with bilateral white matter injury and a profound or central pattern with transient and reversible lesions with decreased diffusion can be observed. -

Os Odontoideum As a Cause of Cervical Cord Injury in a Patient with Refractory Epilepsy
Case Report Clinics in Surgery Published: 09 Feb, 2021 Os Odontoideum as a Cause of Cervical Cord Injury in a Patient with Refractory Epilepsy Shohei Kusabiraki1, Eiji Nakagawa1*, Takashi Saito1, Yutaro Takayama2, Keiya Iijima2, Masaki Iwasaki2, Ayano Matsui3 and Tetsuya Abe4 1Department of Child Neurology, National Center Hospital, NCNP, Japan 2Department of Neurosurgery, National Center Hospital, Japan 3Department of Orthopedics, National Center Hospital, Japan 4Department of Orthopedic Surgery, University of Tsukuba, Japan Abstract Os odontoideum is an anomaly of the second cervical vertebrae in which the odontoid process is separated from the body of the axis. Traumatic injury or congenital fusion failure is thought to be the etiology. The clinical symptoms are variable from cervical pain, torticollis, and myelopathy and vertebrobasilar ischemia. Os odontoideum can cause instability of the neck, and neck injuries can cause life-threatening complications. In this report, we present the case of a 15-year-old girl with refractory epilepsy who developed quadriparesis after a fall and hit to the forehead while traveling. Although the symptoms improved, weakness in her right upper limb persisted at 2 months after the fall. Imaging studies revealed Os odontoideum. Based on her medical history, the recent head trauma due to epileptic seizures accompanied by atlantoaxial instability was considered to result in cervical compression and spinal damage. She was at a high risk of sudden death due to recurrent seizures and cervical injury; therefore, -

The Role of Inhibitory Interneurons in a Model Od Developmental Epilepsy
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2007 The Role of Inhibitory Interneurons in a Model od Developmental Epilepsy Patrick James Wolfgang Virginia Commonwealth University Follow this and additional works at: https://scholarscompass.vcu.edu/etd Part of the Nervous System Commons © The Author Downloaded from https://scholarscompass.vcu.edu/etd/1145 This Thesis is brought to you for free and open access by the Graduate School at VCU Scholars Compass. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. © Patrick James Wolfgang 2007 All Rights Reserved THE ROLE OF INHIBITORY INTERNEURONS IN A MODEL OF DEVELOPMENTAL EPILEPSY A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Anatomy and Neurobiology at Virginia Commonwealth University. by PATRICK JAMES WOLFGANG BA Environmental Science, University of Virginia, 2005 Director: KIMBERLE JACOBS ASSISTANT PROFESSOR ANATOMY AND NEUROBIOLOGY Virginia Commonwealth University Richmond, Virginia August, 2007 ii Acknowledgement First, I would like to thank my parents for their support over the last 25 years. I would also like to thank Dr. Jacobs for her support and guidance during the year. I would also like to extend a sincere thanks to Amanda Lynn George (AKA Mandy 5 or the MD/PhDbot) for keeping me sane as the year progressed. I expect to see your name quite a bit in future publications. I would also like to thank everyone else who has supported me over the years: Michael, Katie, Matthew, Matt, Rob, Hatcher, Tommy, The other Tommy, Patrick, Lauren, Teo, Cara, Shep, Alex, Jamie, Malcolm, Kelly, Shep, Moo, Markowitz, Matt, Jordan, Tina, Kim, Anya, Melissa, Lynn, Sue, all of my teachers, Bam Bam, Grace, the whole Mendez family, the Longos, the Estes, Ms. -

State of the Art &&&&&&&&&&&&&& Neuroimaging and the Timing of Fetal and Neonatal Brain Injury
State of the Art &&&&&&&&&&&&&& Neuroimaging and the Timing of Fetal and Neonatal Brain Injury Patrick D. Barnes, MD modalities provide spatial resolution based upon physiologic or metabolic data. Some modalities may actually be considered to provide both structural and functional information. Ultrasonography (US) Current and advanced structural and functional neuroimaging techniques is primarily a structural imaging modality with some functional 1±11 are presented along with guidelines for utilization and principles of imaging capabilities (e.g., Doppler ÐFigure 1). It is readily accessible, diagnosis in fetal and neonatal central nervous system abnormalities. portable, fast, real time and multiplanar. It less expensive than other Pattern of injury, timing issues, and differential diagnosis are addressed with cross-sectional modalities and relatively noninvasive (nonionizing emphasis on neurovascular disease. Ultrasonography and computed radiation). It requires no contrast agent and infrequently needs tomography provide relatively rapid and important screening information patient sedation. The resolving power of US is based on variations in regarding gross macrostructural abnormalities. However, current and acoustic reflectance of tissues. Its diagnostic effectiveness, however, is advanced MRI techniques often provide more definitive macrostructural, primarily dependent upon the skill and experience of the operator and microstructural, and functional imaging information. interpreter. Also, US requires a window or path unimpeded by bone -

Neonatal Hypoglycemia
096_Valk_Neonatal_Hypoglyc 08.04.2005 16:55 Uhr Seite 749 Chapter 96 Neonatal Hypoglycemia R.J.Vermeulen, J.Valk 96.1 Clinical and Laboratory Findings ronal damage and histochemical differences. Hypo- glycemia leads to reduced concentrations of pyruvate Neonatal hypoglycemia is a condition that frequently and lactate and diminishes the production of protons occurs in sick newborns. The symptomatology in the by the Krebs cycle, resulting in tissue alkalosis, while acute stage can range from agitation, somnolence, hypoxia–ischemia is characterized by elevated lactate and epileptic seizures to status epilepticus and coma. and acidosis. In contrast to hypoxia–ischemia, hypo- Several groups of neonates are at risk of hypo- glycemia does not lead to pannecrosis,but to selective glycemia because of a lack of glucose reserves (dys- neuronal necrosis. This neuronal necrosis involves maturity and prematurity) or an increased need for the cerebral cortex, the hippocampus, the caudate glucose related to high weight and maternal diabetes. nucleus,and sometimes the spinal cord.In contrast to The causes of severe neonatal hypoglycemia are hypoxia–ischemia, the brain stem and the cerebellum diverse and can be separated in three large groups: are spared in hypoglycemia. In addition, the cortical hyperinsulinism (Beckwith–Wiedemann syndrome, neuronal necrosis is more superficial in hypo- nesidioblastosis–adenoma spectrum, hyperplasia of glycemia, whereas the middle cortical layers are pref- the pancreas, leucine sensitivity), endocrine deficien- erentially targeted by hypoxia–ischemia. Axon-spar- cies (cortisol deficiency, growth hormone deficiency, ing parenchymal lesions with selective dendritic glucagon deficiency, hypothyroidism, panhypopitu- swellings are often considered a hallmark of hypo- itarism), and hereditary metabolic defects (defects in glycemic damage. -

Postnatal and Perinatal Trauma. Following Two Introductory
DOCUMENT RESUME ED 047 452 EC 031 5g4 AUTHOR Angle, Carol R., Ed.; Bering, Edgar A., Jr., Pd. TITLE Physical Trauma as an Etiological Agent in Mental Retardation. INSTITUTION National Inst. of Neurological Diseases and Stroke. (DHEW) , Bethesda, Md.; Nebraska Univ., Lincoln. Coll. of Medicine. PUB DATE 70. NOTE 311p.; Proceedings of a Conference on the Etiology of Mental Retardation (Omaha, Nebraska, October 13-16, 1968) AVAILABLE FROM Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402 ($3.75) EDRS PRICE EDRS Price MF-$0.65 HC Not Available from EDRS. DESCRIPTORS Conference Reports, Etiology, *Exceptional Child Research, Incidence, *Infancy, *Injuries, *Medical Research, *Mental Retardation, Neurological Organization, Neurology, Pathology, Premature Infants, Prenatal Influences IDENTIFIERS Pediatrics ABSTRACT The conference on Physical Trauma as a Cause of Mental Retardation dealt with two major areas of etiological concern - postnatal and perinatal trauma. Following two introductory statements on the problem of and issues related to mental retardation (MR) after early trauma to the brain, five papers on the epidemiology of head trauma cover pathological aspects, terminal hemorrhages in the brain wall of neonates, postmortem neuropathologic findings in birth-injured patients, and epidemiological studies. Nine papers report perinatal studies on such topics as obstetric history, obstetric trauma, fetal head position, maternal pelvic size, birth position, intrapartum uterine contractions, and related fetal head compression and heart rate changes. Five special studies of the developing brain concern trauma during labor, trauma to neck vessels, the immature train's reaction to injury, partial brain removal in infant rats, and cerebral ablation in infant monkeys. The following aspects of the premature infant are discussed in relation to MR in five papers: intracranial hemorrhage, CNS damage, clinical evaluation, EEG and subsequent development, and hematologic factors. -

Cortical Ischaemic Patterns in Term Partial-Prolonged Hypoxic
Chacko et al. Insights into Imaging (2020) 11:53 https://doi.org/10.1186/s13244-020-00857-8 Insights into Imaging EDUCATIONAL REVIEW Open Access Cortical ischaemic patterns in term partial- prolonged hypoxic-ischaemic injury—the inter-arterial watershed demonstrated through atrophy, ulegyria and signal change on delayed MRI scans in children with cerebral palsy Anith Chacko1*, Savvas Andronikou1,2, Ali Mian2, Fabrício Guimarães Gonçalves2, Schadie Vedajallam1 and Ngoc Jade Thai1 Abstract The inter-arterial watershed zone in neonates is a geographic area without discernible anatomic boundaries and difficult to demarcate and usually not featured in atlases. Schematics currently used to depict the areas are not based on any prior anatomic mapping, compared to adults. Magnetic resonance imaging (MRI) of neonates in the acute to subacute phase with suspected hypoxic-ischaemic injury (HII) can demonstrate signal abnormality and restricted diffusion in the cortical and subcortical parenchyma of the watershed regions. In the chronic stage of partial-prolonged hypoxic-ischaemic injury, atrophy and ulegyria can make the watershed zone more conspicuous as a region. Our aim is to use images extracted from a sizable medicolegal database (approximately 2000 cases), of delayed MRI scans in children with cerebral palsy, to demonstrate the watershed region. To achieve this, we have selected cases diagnosed on imaging as having sustained a term pattern of partial-prolonged HII affecting the hemispheric cortex, based on the presence of bilateral, symmetric atrophy with ulegyria. From these, we have identified those patients demonstrating injury along the whole watershed continuum as well as those demonstrating selective anterior or posterior watershed predominant injury for demonstration. -

Table of Contents
Table of Contents Introduction: Normal Development and Basic Reactions 1 1. Gross and Microscopic Development of the Central Nervous System .... 2 Timing of Early Embryonal Landmarks 2. — Mass Growth of the Brain 3. — Cerebral Gyri 4. — Lamination of Cerebral Cortex 4. — Superficial Granular Layer 5. — Cells of Cajal-Retzius 5. — Ammon's Horn 5. — Periventricular Germinal Layer 6. — Volume of White Matter 7. — Myelination Gliosis and Development of Astrocytes 7. — Myelination 8. — Regional Timing of Myelination 8. — Basal Ganglia 9. — Mineralization of Cerebral Tissue 9. — Ventricular System 10. — Brain Stem Nuclei 12. — Melanization of Nuclei 12. — Cerebellum 12. — Spinal Cord 16. — References 17. 2. Some Features of Basic Reactions Characteristic for Immature Nervous Tissue 21 Reaction of Immature Nervous Tissue to Necrosis 21. — Age-Dependent Variation in Anoxic Tissue Damage 21. — Anoxic Neuronal Necrosis 21. — Myelination Gliosis 22. — Reactive Gliosis 22. — Fibrillary Gliosis 23. — Differentiation of Glia in the Germinal Layer 23. — Metabolic Astrocytosis 23. — Macrophage Responses 24. — References 24. First Part: Acquired Lesions in Newborns and Infants 27 3. Porencephaly, Hydranencephaly, Multicystic Encephalopathy 28 Porencephaly 28. — Hydranencephaly 31. — Basket Brains 34. — Hydranen cephaly or Porencephaly Related to Fetal Infections 35. — Hydranencephaly with Proliferative Vasculopathy 35. — Cavitated Cerebral Lesions in Twins 35. — Multicystic Encephalopathy 36. — Global Hemispheric Necrosis in Infants 38. — Pathogenetic Considerations 39. — References 40. 4. Hemorrhages in Asphyxiated Premature Infants 44 Subependymal and Intraventricular Hemorrhages 45. — Choroid Plexus Hemorrhages 47. — Subarachnoid Hemorrhages 49. — Subpial Hemorrhages 49. — Hemorrhages into the Falx 50. — Cerebellar Hemorrhages 50. — Hemorrhages at Other Sites 51. — Residual Lesions 52. — Posthemorrhagic Hydrocephalus 53. — Other Residua 54. -

The Pathology of Magnetic-Resonance-Imaging
Modern Pathology (2013) 26, 1051–1058 & 2013 USCAP, Inc. All rights reserved 0893-3952/13 $32.00 1051 The pathology of magnetic-resonance- imaging-negative epilepsy Z Irene Wang1, Andreas V Alexopoulos1, Stephen E Jones2, Zeenat Jaisani3, Imad M Najm1 and Richard A Prayson4 1Cleveland Clinic Epilepsy Center, Cleveland, OH, USA; 2Mellen Imaging Center, Cleveland Clinic, Cleveland, OH, USA; 3Sanford USD Medical Center, Sioux Falls, SD, USA and 4Anatomic Pathology, Cleveland Clinic Foundation, Cleveland, OH, USA Patients with magnetic-resonance-imaging (MRI)-negative (or ‘nonlesional’) pharmacoresistant focal epilepsy are the most challenging group undergoing presurgical evaluation. Few large-scale studies have systematically reviewed the pathological substrates underlying MRI-negative epilepsies. In the current study, histopatholo- gical specimens were retrospectively reviewed from MRI-negative epilepsy patients (n ¼ 95, mean age ¼ 30 years, 50% female subjects). Focal cortical dysplasia cases were classified according to the International League Against Epilepsy (ILAE) and Palmini et al classifications. The most common pathologies found in this MRI-negative cohort included: focal cortical dysplasia (n ¼ 43, 45%), gliosis (n ¼ 21, 22%), hamartia þ gliosis (n ¼ 12, 13%), and hippocampal sclerosis (n ¼ 9, 9%). The majority of focal cortical dysplasia were ILAE type I (n ¼ 37) or Palmini type I (n ¼ 39). Seven patients had no identifiable pathological abnormalities. The existence of positive pathology was not significantly associated with age or temporal/extratemporal resection. Follow-up data post surgery was available in 90 patients; 63 (70%) and 57 (63%) attained seizure freedom at 6 and 12 months, respectively. The finding of positive pathology was significantly associated with seizure-free outcome at 6 months (P ¼ 0.035), but not at 12 months. -

Curved Reformat of the Paediatric Brain MRI Into a 'Flat-Earth Map'
Pediatr Radiol DOI 10.1007/s00247-016-3638-3 TECHNICAL INNOVATION Curved reformat of the paediatric brain MRI into a ‘flat-earth map’—standardised method for demonstrating cortical surface atrophy resulting from hypoxic–ischaemic encephalopathy Ewan Simpson1 & Savvas Andronikou1,2 & Schadie Vedajallam2 & Anith Chacko 2 & Ngoc Jade Thai2 Received: 18 December 2015 /Revised: 23 March 2016 /Accepted: 4 May 2016 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract Hypoxic–ischaemic encephalopathy is optimally Introduction imaged with brain MRI in the neonatal period. However neu- roimaging is often also performed later in childhood (e.g., MRI of the brain in term neonates with hypoxic–ischaemic when parents seek compensation in cases of alleged birth as- encephalopathy reveals damage to the cerebral cortex with phyxia). We describe a standardised technique for creating characteristic morphology (i.e. ulegyria) in characteristic loca- two curved reconstructions of the cortical surface to show tions, depending on the severity and duration of the insult [1]. the characteristic surface changes of hypoxic–ischaemic en- Communicating the bilateral symmetrical geographic involve- cephalopathy in children imaged after the neonatal period. ment of the brain to parents and the legal fraternity contesting The technique was applied for 10 cases of hypoxic–ischaemic compensation rights using standard cross-sectional images is encephalopathy and also for age-matched healthy children to challenging. An overview map of the whole brain surface assess the visibility of characteristic features of hypoxic–isch- generated from a curved reconstruction of the MR images aemic encephalopathy. In the abnormal brains, fissural or may be useful for demonstrating the damage in such scenarios.