State of the Art &&&&&&&&&&&&&& Neuroimaging and the Timing of Fetal and Neonatal Brain Injury
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
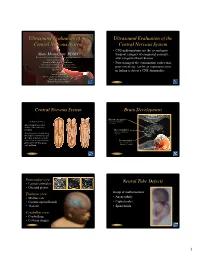
Ultrasound Evaluation of the Central Nervous System
Ultrasound Evaluation of the Ultrasound Evaluation of the Central Nervous System Central Nervous System ••CNSCNS malformations are the second most Mani Montazemi, RDMS frequent category of congenital anomaly, Director of Ultrasound Education & Quality Assurancee after congenital heart disease Baylor College of Medicine Division of Maternal-Fetal Medicine ••PoorPoor timing of the examination, rather than Department of Obstetrics and Gynecology Texas Children’s Hospital, Pavilion for Women poor sensitivity, can be an important factor Houston Texas & in failing to detect a CNS abnormality Clinical Instructor Thomas Jefferson University Hospital Radiology Department Fetal Head Philadelphia, Pennsylvania Fetal Head Central Nervous System Brain Development 9 -13 weeks Rhombencephalon 5th Menstrual Week •Gives rise to hindbrain •4th ventricle Arises from the posterior surface of the embryonic ectoderm Mesencephalon •Gives rise to midbrain A small groove is found along •Aqueduct the midline of the embryo and the edges of this groove fold over to form a neuro tube that Prosencephalon gives rise to the fetal spinal •Gives rise to forebrain rd cord and brain •Lateral & 3 ventricles Fetal Head Fetal Head Ventricular view Neural Tube Defects ••LateralLateral ventricles ••ChoroidChoroid plexus Group of malformations: Thalamic view • Anencephaly ••MidlineMidline falx •Anencephaly ••CavumCavum septiseptipellucidi pellucidi ••CephalocelesCephaloceles ••ThalamiThalami ••SpinaSpina bifida Cerebellar view ••CerebellumCerebellum ••CisternaCisterna magna Fetal -
Introduction to Neuroimaging
Introduction to Neuroimaging Aaron S. Field, MD, PhD Assistant Professor of Radiology Neuroradiology Section University of Wisconsin–Madison Updated 7/17/07 Neuroimaging Modalities Radiography (X-Ray) Magnetic Resonance (MR) Fluoroscopy (guided procedures) • MR Angiography/Venography (MRA/MRV) • Angiography • Diffusion and Diffusion Tensor • Diagnostic MR • Interventional • Perfusion MR • Myelography • MR Spectroscopy (MRS) Ultrasound (US) • Functional MR (fMRI) • Gray-Scale Nuclear Medicine ―Duplex‖ • Color Doppler • Single Photon Emission Computed Tomography (SPECT) Computed Tomography (CT) • Positron Emission Tomography • CT Angiography (CTA) (PET) • Perfusion CT • CT Myelography Radiography (X-Ray) Radiography (X-Ray) Primarily used for spine: • Trauma • Degenerative Dz • Post-op Fluoroscopy (Real-Time X-Ray) Fluoro-guided procedures: • Angiography • Myelography Fluoroscopy (Real-Time X-Ray) Fluoroscopy (Real-Time X-Ray) Digital Subtraction Angiography Fluoroscopy (Real-Time X-Ray) Digital Subtraction Angiography Digital Subtraction Angiography Indications: • Aneurysms, vascular malformations and fistulae • Vessel stenosis, thrombosis, dissection, pseudoaneurysm • Stenting, embolization, thrombolysis (mechanical and pharmacologic) Advantages: • Ability to intervene • Time-resolved blood flow dynamics (arterial, capillary, venous phases) • High spatial and temporal resolution Disadvantages: • Invasive, risk of vascular injury and stroke • Iodinated contrast and ionizing radiation Fluoroscopy (Real-Time X-Ray) Myelography Lumbar or -

Myelography in the Assessment of Degenerative Lumbar Scoliosis And
https://doi.org/10.14245/kjs.2017.14.4.133 KJS Print ISSN 1738-2262 On-line ISSN 2093-6729 CLINICAL ARTICLE Korean J Spine 14(4):133-138, 2017 www.e-kjs.org Myelography in the Assessment of Degenerative Lumbar Scoliosis and Its Influence on Surgical Management George McKay, Objective: Myelography has been shown to highlight foraminal and lateral recess stenosis more Peter Alexander Torrie, readily than computed tomography (CT) or magnetic resonance imaging (MRI). It also has the Wendy Bertram, advantage of providing dynamic assessment of stenosis in the loaded spine. The advent of weight-bearing MRI may go some way towards improving assessment of the loaded spine Priyan Landham, and is less invasive, however availability remains limited. This study evaluates the potential Stephen Morris, role of myelography and its impact upon surgical decision making. John Hutchinson, Methods: Of 270 patients undergoing myelography during 2006-2009, a period representing Roland Watura, peak utilisation of this imaging modality in our unit, we identified 21 patients with degenerative Ian Harding scoliosis who fulfilled our inclusion criteria. An operative plan was formulated by our senior author based initially on interpretation of an MRI scan. Subsequent myelogram and CT myelogram Department of Spinal Surgery, investigations were scrutinised, with any additional abnormalities noted and whether these im- Southmead Hospital, Bristol, United pacted upon the operative plan. Kingdom Results: From our 21 patients, 18 (85.7%) had myelographic findings not identified on MRI. Of Corresponding Author: note, in 4 patients, supine CT myelography yielded additional information when compared to George McKay supine MRI in the same patients. -

Paternal Factors and Schizophrenia Risk: De Novo Mutations and Imprinting
Paternal Factors and Schizophrenia Risk: De Novo Mutations and Imprinting by Dolores Malaspina Downloaded from https://academic.oup.com/schizophreniabulletin/article/27/3/379/1835092 by guest on 23 September 2021 Abstract (Impagnatiello et al. 1998; Kao et al. 1998), but there is no consensus that any particular gene plays a meaning- There is a strong genetic component for schizophrenia ful role in the etiology of schizophrenia (Hyman 2000). risk, but it is unclear how the illness is maintained in Some of the obstacles in genetic research in schiz- the population given the significantly reduced fertility ophrenia are those of any complex disorder, and include of those with the disorder. One possibility is that new incomplete penetrance, polygenic interaction (epista- mutations occur in schizophrenia vulnerability genes. sis), diagnostic instability, and variable expressivity. If so, then those with schizophrenia may have older Schizophrenia also does not show a clear Mendelian fathers, because advancing paternal age is the major inheritance pattern, although segregation analyses have source of new mutations in humans. This review variably supported dominant, recessive, additive, sex- describes several neurodevelopmental disorders that linked, and oligogenic inheritance (Book 1953; Slater have been associated with de novo mutations in the 1958; Garrone 1962; Elston and Campbell 1970; Slater paternal germ line and reviews data linking increased and Cowie 1971; Karlsson 1972; Stewart et al. 1980; schizophrenia risk with older fathers. Several genetic Risch 1990a, 1990fc; reviewed by Kendler and Diehl mechanisms that could explain this association are 1993). Furthermore, both nonallelic (Kaufmann et al. proposed, including paternal germ line mutations, 1998) and etiologic heterogeneity (Malaspina et al. -

Bhagwan Moorjani, MD, FAAP, FAAN • Requires Knowledge of Normal CNS Developmental (I.E
1/16/2012 Neuroimaging in Childhood • Neuroimaging issues are distinct from Pediatric Neuroimaging in adults Neurometabolic-degenerative disorder • Sedation/anesthesia and Epilepsy • Motion artifacts Bhagwan Moorjani, MD, FAAP, FAAN • Requires knowledge of normal CNS developmental (i.e. myelin maturation) • Contrast media • Parental anxiety Diagnostic Approach Neuroimaging in Epilepsy • Age of onset • Peak incidence in childhood • Static vs Progressive • Occurs as a co-morbid condition in many – Look for treatable causes pediatric disorders (birth injury, – Do not overlook abuse, Manchausen if all is negative dysmorphism, chromosomal anomalies, • Phenotype presence (syndromic, HC, NCS, developmental delays/regression) systemic involvement) • Predominant symptom (epilepsy, DD, • Many neurologic disorders in children weakness/motor, psychomotor regression, have the same chief complaint cognitive/dementia) 1 1/16/2012 Congenital Malformation • Characterized by their anatomic features • Broad categories: based on embryogenesis – Stage 1: Dorsal Induction: Formation and closure of the neural tube. (Weeks 3-4) – Stage 2: Ventral Induction: Formation of the brain segments and face. (Weeks 5-10) – Stage 3: Migration and Histogenesis: (Months 2-5) – Stage 4: Myelination: (5-15 months; matures by 3 years) Dandy Walker Malformation Dandy walker • Criteria: – high position of tentorium – dysgenesis/agenesis of vermis – cystic dilatation of fourth ventricle • commonly associated features: – hypoplasia of cerebellum – scalloping of inner table of occipital bone • associated abnormalities: – hydrocephalus 75% – dysgenesis of corpus callosum 25% – heterotropia 10% 2 1/16/2012 Etiology of Epilepsy: Developmental and Genetic Classification of Gray Matter Heterotropia Cortical Dysplasia 1. Secondary to abnormal neuronal and • displaced masses of nerve cells • Subependymal glial proliferation/apoptosis (gray matter) heterotropia (most • most common: small nest common) 2. -

Approach to Brain Malformations
Approach to Brain Malformations A General Imaging Approach to Brain CSF spaces. This is the basis for development of the Dandy- Malformations Walker malformation; it requires abnormal development of the cerebellum itself and of the overlying leptomeninges. Whenever an infant or child is referred for imaging because of Looking at the midline image also gives an idea of the relative either seizures or delayed development, the possibility of a head size through assessment of the craniofacial ratio. In the brain malformation should be carefully investigated. If the normal neonate, the ratio of the cranial vault to the face on child appears dysmorphic in any way (low-set ears, abnormal midline images is 5:1 or 6:1. By 2 years, it should be 2.5:1, and facies, hypotelorism), the likelihood of an underlying brain by 10 years, it should be about 1.5:1. malformation is even higher, but a normal appearance is no guarantee of a normal brain. In all such cases, imaging should After looking at the midline, evaluate the brain from outside be geared toward showing a structural abnormality. The to inside. Start with the cerebral cortex. Is the thickness imaging sequences should maximize contrast between gray normal (2-3 mm)? If it is too thick, think of pachygyria or matter and white matter, have high spatial resolution, and be polymicrogyria. Is the cortical white matter junction smooth or acquired as volumetric data whenever possible so that images irregular? If it is irregular, think of polymicrogyria or Brain: Pathology-Based Diagnoses can be reformatted in any plane or as a surface rendering. -

A Anencephaly
Glossary of Birth Anomaly Terms: A Anencephaly: A deadly birth anomaly where most of the brain and skull did not form. Anomaly: Any part of the body or chromosomes that has an unusual or irregular structure. Aortic valve stenosis: The aortic valve controls the flow of blood from the left ventricle of the heart to the aorta, which takes the blood to the rest of the body. If there is stenosis of this valve, the valve has space for blood to flow through, but it is too narrow. Atresia: Lack of an opening where there should be one. Atrial septal defect: An opening in the wall (septum) that separates the left and right top chambers (atria) of the heart. A hole can vary in size and may close on its own or may require surgery. Atrioventricular septal defect (endocardial cushion defect): A defect in both the lower portion of the atrial septum and the upper portion of the ventricular septum. Together, these defects make a large opening (canal) in the middle part of the heart. Aniridia (an-i-rid-e-a): An eye anomaly where the colored part of the eye (called the iris) is partly or totally missing. It usually affects both eyes. Other parts of the eye can also be formed incorrectly. The effects on children’s ability to see can range from mild problems to blindness. To learn more about aniridia, go to the U.S. National Library of Medicine website. Anophthalmia/microphthalmia (an-oph-thal-mia/mi-croph-thal-mia): Birth anomalies of the eyes. In anophthalmia, a baby is born without one or both eyes. -

Mutation in Genes FBN1, AKT1, and LMNA: Marfan Syndrome, Proteus Syndrome, and Progeria Share Common Systemic Involvement
Review Mutation in Genes FBN1, AKT1, and LMNA: Marfan Syndrome, Proteus Syndrome, and Progeria Share Common Systemic Involvement Tonmoy Biswas.1 Abstract Genetic mutations are becoming more deleterious day by day. Mutations of Genes named FBN1, AKT1, LMNA result specific protein malfunction that in turn commonly cause Marfan syndrome, Proteus syndrome, and Progeria, respectively. Articles about these conditions have been reviewed in PubMed and Google scholar with a view to finding relevant clinical features. Precise keywords have been used in search for systemic involvement of FBN1, AKT1, and LMNA gene mutations. It has been found that Marfan syndrome, Proteus syndrome, and Progeria commonly affected musculo-skeletal system, cardiovascular system, eye, and nervous system. Not only all of them shared identical systemic involvement, but also caused several very specific anomalies in various parts of the body. In spite of having some individual signs and symptoms, the mutual manifestations were worth mentio- ning. Moreover, all the features of the mutations of all three responsible genes had been co-related and systemically mentioned in this review. There can be some mutual properties of the genes FBN1, AKT1, and LMNA or in their corresponding proteins that result in the same presentations. This study may progress vision of knowledge regarding risk factors, patho-physiology, and management of these conditions, and relation to other mutations. Keywords: Genetic mutation; Marfan syndrome; Proteus syndrome; Progeria; Gene FBN1; Gene AKT1; Gene LMNA; Musculo-skeletal system; Cardiovascular system; Eye; Nervous system (Source: MeSH, NLM). Introduction Records in human mutation databases are increasing day by 5 About the author: Tonmoy The haploid human genome consists of 3 billion nucleotides day. -
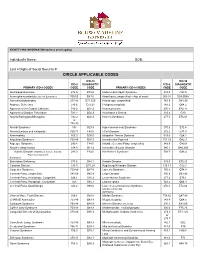
Circle Applicable Codes
IDENTIFYING INFORMATION (please print legibly) Individual’s Name: DOB: Last 4 Digits of Social Security #: CIRCLE APPLICABLE CODES ICD-10 ICD-10 ICD-9 DIAGNOSTIC ICD-9 DIAGNOSTIC PRIMARY ICD-9 CODES CODE CODE PRIMARY ICD-9 CODES CODE CODE Abetalipoproteinemia 272.5 E78.6 Hallervorden-Spatz Syndrome 333.0 G23.0 Acrocephalosyndactyly (Apert’s Syndrome) 755.55 Q87.0 Head Injury, unspecified – Age of onset: 959.01 S09.90XA Adrenaleukodystrophy 277.86 E71.529 Hemiplegia, unspecified 342.9 G81.90 Arginase Deficiency 270.6 E72.21 Holoprosencephaly 742.2 Q04.2 Agenesis of the Corpus Callosum 742.2 Q04.3 Homocystinuria 270.4 E72.11 Agenesis of Septum Pellucidum 742.2 Q04.3 Huntington’s Chorea 333.4 G10 Argyria/Pachygyria/Microgyria 742.2 Q04.3 Hurler’s Syndrome 277.5 E76.01 or 758.33 Aicardi Syndrome 333 G23.8 Hyperammonemia Syndrome 270.6 E72.4 Alcohol Embryo and Fetopathy 760.71 F84.5 I-Cell Disease 272.2 E77.0 Anencephaly 655.0 Q00.0 Idiopathic Torsion Dystonia 333.6 G24.1 Angelman Syndrome 759.89 Q93.5 Incontinentia Pigmenti 757.33 Q82.3 Asperger Syndrome 299.8 F84.5 Infantile Cerebral Palsy, unspecified 343.9 G80.9 Ataxia-Telangiectasia 334.8 G11.3 Intractable Seizure Disorder 345.1 G40.309 Autistic Disorder (Childhood Autism, Infantile 299.0 F84.0 Klinefelter’s Syndrome 758.7 Q98.4 Psychosis, Kanner’s Syndrome) Biotinidase Deficiency 277.6 D84.1 Krabbe Disease 333.0 E75.23 Canavan Disease 330.0 E75.29 Kugelberg-Welander Disease 335.11 G12.1 Carpenter Syndrome 759.89 Q87.0 Larsen’s Syndrome 755.8 Q74.8 Cerebral Palsy, unspecified 343.69 G80.9 -

2018 Etiologies by Frequencies
2018 Etiologies in Order of Frequency by Category Hereditary Syndromes and Disorders Count CHARGE Syndrome 958 Down syndrome (Trisomy 21 syndrome) 308 Usher I syndrome 252 Stickler syndrome 130 Dandy Walker syndrome 119 Cornelia de Lange 102 Goldenhar syndrome 98 Usher II syndrome 83 Wolf-Hirschhorn syndrome (Trisomy 4p) 68 Trisomy 13 (Trisomy 13-15, Patau syndrome) 60 Pierre-Robin syndrome 57 Moebius syndrome 55 Trisomy 18 (Edwards syndrome) 52 Norrie disease 38 Leber congenital amaurosis 35 Chromosome 18, Ring 18 31 Aicardi syndrome 29 Alstrom syndrome 27 Pfieffer syndrome 27 Treacher Collins syndrome 27 Waardenburg syndrome 27 Marshall syndrome 25 Refsum syndrome 21 Cri du chat syndrome (Chromosome 5p- synd) 16 Bardet-Biedl syndrome (Laurence Moon-Biedl) 15 Hurler syndrome (MPS I-H) 15 Crouzon syndrome (Craniofacial Dysotosis) 13 NF1 - Neurofibromatosis (von Recklinghausen dis) 13 Kniest Dysplasia 12 Turner syndrome 11 Usher III syndrome 10 Cockayne syndrome 9 Apert syndrome/Acrocephalosyndactyly, Type 1 8 Leigh Disease 8 Alport syndrome 6 Monosomy 10p 6 NF2 - Bilateral Acoustic Neurofibromatosis 6 Batten disease 5 Kearns-Sayre syndrome 5 Klippel-Feil sequence 5 Hereditary Syndromes and Disorders Count Prader-Willi 5 Sturge-Weber syndrome 5 Marfan syndrome 3 Hand-Schuller-Christian (Histiocytosis X) 2 Hunter Syndrome (MPS II) 2 Maroteaux-Lamy syndrome (MPS VI) 2 Morquio syndrome (MPS IV-B) 2 Optico-Cochleo-Dentate Degeneration 2 Smith-Lemli-Opitz (SLO) syndrome 2 Wildervanck syndrome 2 Herpes-Zoster (or Hunt) 1 Vogt-Koyanagi-Harada -

Pushing the Limits of Prenatal Ultrasound: a Case of Dorsal Dermal Sinus Associated with an Overt Arnold–Chiari Malformation and a 3Q Duplication
reproductive medicine Case Report Pushing the Limits of Prenatal Ultrasound: A Case of Dorsal Dermal Sinus Associated with an Overt Arnold–Chiari Malformation and a 3q Duplication Olivier Leroij 1, Lennart Van der Veeken 2,*, Bettina Blaumeiser 3 and Katrien Janssens 3 1 Faculty of Medicine, University of Antwerp, 2610 Wilrijk, Belgium; [email protected] 2 Department of Obstetrics and Gynaecology, University Hospital Antwerp, 2650 Edegem, Belgium 3 Department of Medical Genetics, University Hospital and University of Antwerp, 2650 Edegem, Belgium; [email protected] (B.B.); [email protected] (K.J.) * Correspondence: [email protected] Abstract: We present a case of a fetus with cranial abnormalities typical of open spina bifida but with an intact spine shown on both ultrasound and fetal MRI. Expert ultrasound examination revealed a very small tract between the spine and the skin, and a postmortem examination confirmed the diagnosis of a dorsal dermal sinus. Genetic analysis found a mosaic 3q23q27 duplication in the form of a marker chromosome. This case emphasizes that meticulous prenatal ultrasound examination has the potential to diagnose even closed subtypes of neural tube defects. Furthermore, with cerebral anomalies suggesting a spina bifida, other imaging techniques together with genetic tests and measurement of alpha-fetoprotein in the amniotic fluid should be performed. Citation: Leroij, O.; Van der Veeken, Keywords: dorsal dermal sinus; Arnold–Chiari anomaly; 3q23q27 duplication; mosaic; marker chro- L.; Blaumeiser, B.; Janssens, K. mosome Pushing the Limits of Prenatal Ultrasound: A Case of Dorsal Dermal Sinus Associated with an Overt Arnold–Chiari Malformation and a 3q 1. -

Imaging Surgical Epilepsy in Children
Childs Nerv Syst (2006) 22:786–809 DOI 10.1007/s00381-006-0132-5 SPECIAL ANNUAL ISSUE Imaging surgical epilepsy in children Charles Raybaud & Manohar Shroff & James T. Rutka & Sylvester H. Chuang Received: 1 February 2006 / Published online: 13 June 2006 # Springer-Verlag 2006 Abstract of the diverse pathologies concerned with epilepsy surgery in Introduction Epilepsy surgery rests heavily upon magnetic the pediatric context is provided with illustrative images. resonance imaging (MRI). Technical developments have brought significantly improved efficacy of MR imaging in Keywords MR imaging . Epilepsy. Ganglioglioma . DNT. detecting and assessing surgical epileptogenic lesions, PXA . FCD . Hypothalamic hamartoma . while more clinical experience has brought better definition Meningioangiomatosis of the pathological groups. Discussion MRI is fairly efficient in identifying develop- mental, epilepsy-associated tumors such as ganglioglioma Introduction (with its variants gangliocytoma and desmoplastic infantile ganglioglioma), the complex, simple and nonspecific forms Epilepsy surgery in children addresses severe refractory of dysembryoplastic neuroepithelial tumor, and the rare epilepsy mainly, which threatens the child’s development, pleomorphic xanthoastrocytoma. The efficacy of MR when a constant epileptogenic focus can be identified from imaging is not as good for the diagnosis of focal cortical the clinical and the electroencephalogram (EEG) data and be dysplasia (FCD), as it does not necessarily correlate with removed. Imaging must demonstrate