GSK Annual Report 2010
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Copyright by Gwendolyn Sarah Kirk 2016
Copyright by Gwendolyn Sarah Kirk 2016 The Dissertation committee for Gwendolyn Sarah Kirk certifies that this is the approved version of the following dissertation: Uncivilized language and aesthetic exclusion: Language, power and film production in Pakistan Committee: _____________________________ Craig Campbell, Co-Supervisor _____________________________ Elizabeth Keating, Co-Supervisor _____________________________ Kamran Ali _____________________________ Patience Epps _____________________________ Ali Khan _____________________________ Kathleen Stewart _____________________________ Anthony Webster Uncivilized language and aesthetic exclusion: Language, power and film production in Pakistan by Gwendolyn Sarah Kirk, B.A.; M.A. Dissertation Presented to the Faculty of the Graduate School of the University of Texas at Austin in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy The University of Texas at Austin December 2016 To my parents Acknowledgements This dissertation would not have been possible first and foremost without the kindness and generosity of the filmmakers I worked with at Evernew Studio. Parvez Rana, Hassan Askari, Z.A. Zulfi, Pappu Samrat, Syed Noor, Babar Butt, and literally everyone else I met in the film industry were welcoming and hospitable beyond what I ever could have hoped or imagined. The cast and crew of Sharabi, in particular, went above and beyond to facilitate my research and make sure I was at all times comfortable and safe and had answers to whatever stupid questions I was asking that day! Along with their kindness, I was privileged to witness their industry, creativity, and perseverance, and I will be eternally inspired by and grateful to them. My committee might seem large at seven members, but all of them have been incredibly helpful and supportive throughout my time in graduate school, and each of them have helped develop different dimensions of this work. -

Glaxosmithkline Bangladesh Limited (GSK)
GlaxoSmithKline Bangladesh Limited (GSK) Recruitment and Selection Process of GlaxoSmithKline Bangladesh Limited: An Evaluation nd Date of Submission: 2 September, 2014 ©Daffodil International University DAFFODIL INTERNATIONAL UNIVERSITY Internship Report On Recruitment and Selection Process of GlaxoSmithKline Bangladesh Limited: An Evaluation Submitted To Dr. Zakir Hossain Dean& Professor Faculty of Business & Economics Daffodil International University Submitted By Ishrat Jahan ID: 131-14-1019 Masters of Business Administration Daffodil International University Date of Submission: 2nd September, 2014 ©Daffodil International University Letter of Transmittal September 2, 2014 To Dr. Zakir Hossain Dean & Professor Faculty of Business and Economics Daffodil International University Dhaka-1205 Subject: Submission of internship report on recruitment and selection process: An evaluation of GlaxoSmithKline Bangladesh Limited Sir, I am highly satisfied to submit my report on recruitment and selection process: an evaluation of GSK. For preparing this report I tried my best to accumulate relevant and upgraded information from available sources. In preparing this report, I tried my level best to make it a complete one and sincerely look forward to any possible correction. I am very glad because you also given the opportunity to prepare this report .I hope that this report will meet the standards of your judgments. Your Sincerely ---------------- Ishrat Jahan ©Daffodil International University i Certificate of the Supervisor This is to certify that the internship report titled ―Recruitment and Selection Process of GlaxoSmithKline Bangladesh Limited: An Evaluation‖, has been prepared by Ms. Ishrat Jahan bearing ID: 131-14-1019 under my supervision, a practical study on GlaxoSmithKline Bangladesh Limited. I think on the basic of declaration Ms. -

University Law College University of the Punjab, Lahore
1 University Law College University of the Punjab, Lahore. Result of Entry Test of LL.B 03 Years Morning/Afternoon Program Session (2018-2019) (Annual System) held on 12-08-2018* Total Marks: 80 Roll No. Name of Candidate Father's Name Marks 1 Nusrat Mushtaq Mushtaq Ahmed 16 2 Usman Waqas Mohammad Raouf 44 3 Ali Hassan Fayyaz Akhtar 25 4 Muneeb Ahmad Saeed Ahmad 44 5 Sajid Ali Muhammad Aslam 30 6 Muhammad Iqbal Irshad Hussain 23 7 Ameen Babar Abid Hussain Babar Absent 8 Mohsin Jamil Muhammad Jamil 24 9 Syeda Dur e Najaf Bukhari Muhammad Noor ul Ain Bukhari Absent 10 Ihtisham Haider Haji Muhammad Afzal 20 11 Muhammad Nazim Saeed Ahmad 30 12 Muhammad Wajid Abdul Waheed 22 13 Ashiq Hussain Ghulam Hussain 14 14 Abdullah Muzafar Ali 35 15 Naoman Khan Muhammad Ramzan 33 16 Abdullah Masaud Masaud Ahmad Yazdani 30 17 Muhammad Hashim Nadeem Mirza Nadeem Akhtar 39 18 Muhammad Afzaal Muhammad Arshad 13 19 Muhammad Hamza Tahir Nazeer Absent 20 Abdul Ghaffar Muhammad Majeed 19 21 Adeel Jan Ashfaq Muhammad Ashfaq 24 22 Rashid Bashir Bashir Ahmad 35 23 Touseef Akhtar Khursheed Muhammad Akhtar 42 24 Usama Nisar Nisar Ahmad 17 25 Haider Ali Zulfiqar Ali 26 26 Zeeshan Ahmed Sultan Ahmed 28 27 Hafiz Muhammad Asim Muhammad Hanif 32 28 Fatima Maqsood Maqsood Ahmad 12 29 Abdul Waheed Haji Musa Khan 16 30 Simran Shakeel Ahmad 28 31 Muhammad Sami Ullah Muhammad Shoaib 35 32 Muhammad Sajjad Shoukat Ali 27 33 Muhammad Usman Iqbal Allah Ditta Urf M. Iqbal 27 34 Noreen Shahzadi Muhammad Ramzan 19 35 Salman Younas Muhammad Younas Malik 25 36 Zain Ul Abidin Muhammad Aslam 21 37 Muhammad Zaigham Faheem Muhammad Arif 32 38 Shahid Ali Asghar Ali 37 39 Abu Bakar Jameel M. -

Annual Report 2013
Annual Report 2013 “ Being active and having a positive outlook on life is what keeps me going every day.” Overview of 2013 “ Our performance in 2013 was defined by remarkable &R D output and further delivery of sustained financial performance for our shareholders.” Please go to page 4 for more More at gsk.com Performance highlights £26.5bn £8.0bn £7.0bn £5.2bn Group turnover Core* operating profit Total operating profit Returned to shareholders 6 112.2p 112.5p 13% Major medicines approved Core* earnings per share Total earnings per share Estimated return on R&D investment 10 6 1st 1st Potential phase III study starts in 2014/15 Potential medicines with phase III data in Access to Medicines Index Pharmaceutical company to sign AllTrials expected 2014/15 campaign for research transparency Front cover story Betty, aged 65, (pictured) has Chronic “ Health is important to me, Obstructive Pulmonary Disease (COPD). She only has 25% lung capacity. This means I try to take care of my she finds even everyday tasks difficult, but medicines and inhaled oxygen allow her to health with all the tools live as normal a life as she can. Betty’s mindset I have and do the best is to stay busy and active, so every week she goes to rehab exercise classes. that I can with it.” COPD is a disease of the lungs that leads to Betty, COPD patient, damaged airways, causing them to become North Carolina, USA narrower and making it harder for air to get in and out. 210 million people around the world are estimated to have COPD. -

A Case Study of Glaxosmithkline Plc )
)l GROWTH THROUGH MERGER: A CASE STUDY OF GLAXOSMITHKLINE PLC ) / BY JUNE WANGARI UNIVERSITY OF NAIR03J LOWER KABETE LIBRARY A MANAGEMENT RESEARCH PROJECT SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE AWARD OF DEGREE OF MASTER OF BUSINESS ADMINISTRATION (MBA), SCHOOL OF BUSINESS, UNIVERSITY OF NAIROBI JULY, 2008 DECLARATION I declare that this project is my original work and has never been presented for academic purposes in any other University. CANDIDATE: JUNE WANGARI DATE .(.91. This research project has been submitted for examination with my approval as the University Supervisor SIGNED. DATE. Prof. Evans Aosa Department Of Business Administration, School Of Business, University Of Nairobi 11 DEDICATION I dedicate this project to my daughter Jemima, who was born within the first year of my post graduate studies. And now that she is in kindergarten, I see that I have instilled in her the love for reading and learning and I trust that she will go very far. iii ACKNOWLEDGEMENT I thank God for seeing me through my studies as I tried to balance between my family, my work and my studies. I wish to acknowledge the contributions that were made in the course of this project by several individuals and organizations. I wish to acknowledge gratefully the following people, whose effort influenced the content and direction of this project. My first thanks go to my Supervisor Prof. Evans Aosa for his constant analytical criticism and encouragement. Thanks a lot. I wish to thank my friends for a lot of support and encouragement to me in pursuit of this goal. -

GSK Q1 Report 2021
Calibrating Precision GSK Pakistan Limited FirstAnnual Quarter Report Report 20212020 CORPORATE INFORMATION As at March 31, 2021 Board of Directors Management Committee Disclosure Committee Mr. Dmytro Oliinyk Ms. Erum Shakir Rahim Ms. Erum Shakir Rahim Chairman Chief Executive Officer Chairperson Ms. Erum Shakir Rahim Mr. Abdul Samad* Mr. Abdul Samad* Chief Executive Officer Chief Financial Officer Member Mr. Abdul Samad* Mehar-e-daraksha Ameer Mr. Dmytro Oliinyk Chief Financial Officer Legal Director Member Ms. Maheen Rahman Dr. Tariq Farooq Mehar-e-daraksha Ameer Independent Director Business Unit Director BU 1 Member Mr. Muneer Kamal Syed Nasir Farid** Company Secretary Independent Director Business Unit Director BU 2 Ms. Mehar-e-daraksha Ameer Mr. Mehmood Mandviwalla Dr. Naved Masoom Ali Non-Executive Director Business Unit Director BU 3 Chief Financial Officer Mr. Mark Dawson Khurram Amjad Mr. Abdul Samad* Non-Executive Director Director Commercial Chief Internal Auditor Excellence & CTC Audit Committee Syed Ahsan Ejaz Dr. Gohar Nayab Khan Mr. Muneer Kamal Regulatory Affairs Cluster Bankers Chairman Head - Pakistan & Iran Citibank NA Mr. Dmytro Oliinyk Mr. Abdul Haseeb Pirzada Deutsche Bank A.G. Member Director Corporate Affairs and Habib Bank Limited Administration Meezan Bank Limited Mr. Mark Dawson Standard Chartered Bank (Pakistan) Ltd Member Mr. Zain Anjum Country Compliance Officer Auditors Mr. Mehmood Mandviwalla Member Dr. Yousuf Hasan Khan Yousuf Adil & Co. Chartered Director Medical Accountants Ms. Maheen Rahman Member Syed Nabigh Raza Alam Legal Advisors Tech Head Hashmi & Hashmi Human Resource Mr. Obaid Siddiqui Faisal, Mahmood Ghani and Co & Remuneration Head of Procurement Legal Consultancy Inc. Committee Mr. Farqaleet Iqbal Registered Office Mr. -

GSK Annual Report 2016
Annual Report Transcending boundaries is in our DNA. This is evident in how we continue to discover, develop and bring to market, new medicines and vaccines to address the unmet needs of healthcare providers and their patients. As a responsible healthcare company, we drive change by adopting new and innovative methodologies, aligned with our values, so that we can better serve the evolving needs of our society and enable more patients to benefit from our medicines. We firmly believe that keeping the patient at the heart of everything we do is integral to our success; inspiring us to continue on this journey and transcend boundaries. GSK Contents 002 026 062 Our Vision, Mission and Core Vaccines Key Operating and Financial Data Values 028 065 004 Primary Care Direct Cash Flow Statement Corporate Information 030 066 006 Specialty Business Unit Horizontal Analysis Financial Highlights 031 067 007 Medical Affairs Vertical Analysis DUPONT Analysis 032 070 008 Awards 2016 Statement of Compliance with the The GSK Story 034 Code of Corporate Governance 010 Global Manufacturing & Supply 072 GSK Expectations (GMS) Review Report to the Members 011 035 073 Strategic Priorities Warehousing and Auditors Report to the Members 012 Distribution (W&D) 074 Company Profile & 036 Balance Sheet Group Structure Environment Health & Safety (EHS) 075 014 Profit and Loss Account Our Behaviour 038 Quality Management System (QMS) 076 015 Cash Flow Statement Organogram 040 Corporate Social Responsibility 077 016 (CSR) Statement of Changes in Equity Geographical Presence -

Uzomis: at All Doses Compared to Those on Placebo
persistent and recurrent headache. The frequency and time to use of these additional WARNINGS treatments were also recorded. ZOMIG* (zolmitriptan) should only be used where a clear diagnosis of migraine Table 1 shows efficacy results for ZOMIG" in 5 placebo-controlled trials, 4 of which were has been established. multicenter. The percentage of patients with pain relief (grade 1/0) at 2 hours after treatment Risk of Myocardial Ischemia ami/or Infarction and Other Adverse Cardiac Events: (the primary endpoint measure) was significantly greater among patients receiving ZOMIG* 2DWB* has beMassccMedwMtmis^ chest siMfrJMkpste and 8ghtoe& UZomis: at all doses compared to those on placebo. In Study 3, which directly compared the 1 mg, which may resemble angina pectoris. Following the use of other 5-HTj 2.5 mg and 5 mg doses, there was a statistically significant greater proportion of patients with agonists, In rare cases these symptoms have been Identified as being the likely zolmitriptan tablets 2.5 mg headache response at 2 and 4 hours in the higher dose groups (2.5 mg or 5 mg) than in the result of coronary vasospasm or myocardial Ischemia. Rare cases of serious 1 mg group. There was no statistically significant difference between the 2.5 mg and 5 mg PHARMACOLOGICAL CLASSIFICATION coronary events or arrhythmia have occurred following use ot S-HTt agonists, dose groups for the primary endpoint measure of pain relief (1/0) at 2 hours, or at any other including ZOMIG*. ZOMIG* should not be given to patients who have documented 5-HTi Receptor Agonist time point measured. -
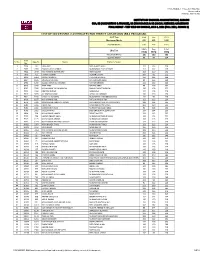
Announced on Monday, July 19, 2021
FINAL RESULT - FALL 2021 ROUND 2 Announced on Monday, July 19, 2021 INSTITUTE OF BUSINESS ADMINISTRATION, KARACHI BBA, BS (ACCOUNTING & FINANCE), BS (ECONOMICS) & BS (SOCIAL SCIENCES) ADMISSIONS FINAL RESULT ‐ TEST HELD ON SUNDAY, JULY 4, 2021 (FALL 2021, ROUND 2) LIST OF SUCCESSFUL CANDIDATES FOR DIRECT ADMISSION (BBA PROGRAM) SAT Test Math Eng TOTAL Maximum Marks 800 800 1600 Cut-Off Marks 600 600 1420 Math Eng Total IBA Test MCQ MCQ MCQ Maximum Marks 180 180 360 Cut-Off Marks 88 88 224 Seat S. No. App No. Name Father's Name No. 1 7904 30 LAIBA RAZI RAZI AHMED JALALI 112 116 228 2 7957 2959 HASSAAN RAZA CHINOY MUHAMMAD RAZA CHINOY 112 132 244 3 7962 3549 MUHAMMAD SHAYAN ARIF ARIF HUSSAIN 152 120 272 4 7979 455 FATIMA RIZWAN RIZWAN SATTAR 160 92 252 5 8000 1464 MOOSA SHERGILL FARZAND SHERGILL 124 124 248 6 8937 1195 ANAUSHEY BATOOL ATTA HUSSAIN SHAH 92 156 248 7 8938 1200 BIZZAL FARHAN ALI MEMON FARHAN MEMON 112 112 224 8 8978 2248 AFRA ABRO NAVEED ABRO 96 136 232 9 8982 2306 MUHAMMAD TALHA MEMON SHAHID PARVEZ MEMON 136 136 272 10 9003 3266 NIRDOSH KUMAR NARAIN NA 120 108 228 11 9017 3635 ALI SHAZ KARMANI IMTIAZ ALI KARMANI 136 100 236 12 9031 1945 SAIFULLAH SOOMRO MUHAMMAD IBRAHIM SOOMRO 132 96 228 13 9469 1187 MUHAMMAD ADIL RAFIQ AHMAD KHAN 112 112 224 14 9579 2321 MOHAMMAD ABDULLAH KUNDI MOHAMMAD ASGHAR KHAN KUNDI 100 124 224 15 9582 2346 ADINA ASIF MALIK MOHAMMAD ASIF 104 120 224 16 9586 2566 SAMAMA BIN ASAD MUHAMMAD ASAD IQBAL 96 128 224 17 9598 2685 SYED ZAFAR ALI SYED SHAUKAT HUSSAIN SHAH 124 104 228 18 9684 526 MUHAMMAD HAMZA -

API Written Confirmation 02-08-2021
MHRA Register of Written Confirmations For UK Active Substance Manufacturers The information published in this document was that held by the MHRA on the date of publication. Please note the site register will be updated on a Monthly basis. Date of Publication: 02/08/2021 Copyright © by the Department of Health and Social Care and MHRA, 10 South Colonnade, Canary Wharf, London E14 4PU T 020 3080 6000 – www.mhra.gov.uk NOTICES The Agency’s register is computerised. Every site and every Written Confirmation has a unique number that should be quoted when enquiries are made. NOTES FOR GUIDANCE GENERAL The Written Confirmations have been generated for UK Active Substance Manufacturing sites to support the export of Active Substances to the EEA. The Written Confirmation Number is a specific number allocated to each site. The Table of Contents contains a link to the relevant Written Confirmation for each company within this document. Table of Contents A NELSON AND COMPANY LIMITED AIR PRODUCTS PLC ALBUMEDIX LIMITED ALLIANCE MEDICAL RADIOPHARMACY LIMITED ALLIANCE MEDICAL RADIOPHARMACY LIMITED ALMAC SCIENCES (SCOTLAND) LIMITED ALMAC SCIENCES LIMITED APTUIT (OXFORD) LIMITED APTUIT (OXFORD) LIMITED ARC TRINOVA LIMITED BASILDON CHEMICAL COMPANY LIMITED BAXTER HEALTHCARE LIMITED BAXTER HEALTHCARE LIMITED BIO PRODUCTS LABORATORY LIMITED BSPG LABORATORIES LIMITED CATALENT MICRON TECHNOLOGIES LIMITED COURTIN & WARNER LIMITED CRODA EUROPE LIMITED CRODA EUROPE LIMITED DR REDDY'S LABORATORIES (EU) LIMITED DSM NUTRITIONAL PRODUCTS (UK) LIMITED EUROAPI UK LIMITED -

OICCI CSR Report 2018-2019
COMBINING THE POWER OF SOCIAL RESPONSIBILITY Corporate Social Responsibility Report 2018-19 03 Foreword CONTENTS 05 OICCI Members’ CSR Impact 06 CSR Footprint – Members’ Participation In Focus Areas 07 CSR Footprint – Geographic Spread of CSR Activities 90 Snapshot of Participants’ CSR Activities 96 Social Sector Partners DISCLAIMER The report has been prepared by the Overseas Investors Chamber of Commerce and Industry (OICCI) based on data/information provided by participating companies. The OICCI is not liable for incorrect representation, if any, relating to a company or its activities. 02 | OICCI FOREWORD The landscape of CSR initiatives and activities is actively supported health and nutrition related initiatives We are pleased to present improving rapidly as the corporate sector in Pakistan has through donations to reputable hospitals, medical care been widely adopting the CSR and Sustainability camps and health awareness campaigns. Infrastructure OICCI members practices and making them permanent feature of the Development was also one of the growing areas of consolidated 2018-19 businesses. The social areas such as education, human interest for 65% of the members who assisted communi- capital development, healthcare, nutrition, environment ties in the vicinity of their respective major operating Corporate Social and infrastructure development are the main focus of the facilities. businesses to reach out to the underprivileged sections of Responsibility (CSR) the population. The readers will be pleased to note that 79% of our member companies also promoted the “OICCI Women” Report, highlighting the We, at OICCI, are privileged to have about 200 leading initiative towards increasing level of Women Empower- foreign investors among our membership who besides ment/Gender Equality. -

UPL Annual Report 2019
Unilever Pakistan Foods Limited Annual Report 2019 Contents Vision & Core Values 02 Company Information 03 Directors’ Profile 04 Chairman's Review 06 Directors’ Report 08 Board Meetings Attendance 14 Board Committee Meetings Held During the Year 14 Performance Indicators for 6 years 16 Statement of Financial Postion - Analysis for 6 years 19 Profit or Loss Account and other Comprehensive 21 Income - Analysis for 6 years Statement of Wealth Generated and Distributed 23 Pattern of Shareholding 24 Statement of Compliance with 26 the Code of Corporate Governance Independent Auditor's Review Report 27 Financial Statements 29 Notice of Annual General Meeting 79 Form of Proxy 85 Dividend Mandate Form 87 Vision Unilever Pakistan Foods Limited - Annual Report 2019 Our vision is to grow our business, while decoupling our environmental footprint from our growth and increasing our positive social impact. Core Values Impeccable Wowing our Integrity Consumers & We are honest, transparent Customers and ethical in our dealings We win the hearts and minds of at all times. our consumers and customers. Living an Demonstrating a Enterprise Culture Passion for Winning We believe in trust, and We deliver what we promise. outstanding teamwork. We value a creative & fun environment. Bringing out the Making a Best in All of Us Better World We are empowered leaders, who We care about and are inspired by new challenges actively contribute to and have a bias for action. the community in which we live. 02 Company Information Unilever Pakistan Foods Limited - Annual Report 2019 Board of Directors Mr. Kamran Y. Mirza Independent Director & Chairman of the Board Mr.