Identifing Priority Ecoregions for Rodent Conservation at the Genus Level
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

13914444D46c0aa91d02e31218
2 Breeding of wild and some domestic animals at regional zoological institutions in 2013 3 РЫБЫ P I S C E S ВОББЕЛОНГООБРАЗНЫЕ ORECTOLOBIFORMES Сем. Азиатские кошачьи акулы (Бамбуковые акулы) – Hemiscyllidae Коричневополосая бамбуковая акула – Chiloscyllium punctatum Brownbanded bambooshark IUCN (NT) Sevastopol 20 ХВОСТОКОЛООБРАЗНЫЕ DASYATIFORMES Сем. Речные хвостоколы – Potamotrygonidae Глазчатый хвостокол (Моторо) – Potamotrygon motoro IUCN (DD) Ocellate river stingray Sevastopol - ? КАРПООБРАЗНЫЕ CYPRINIFORMES Сем. Цитариновые – Citharinidae Серебристый дистиход – Distichodusaffinis (noboli) Silver distichodus Novosibirsk 40 Сем. Пираньевые – Serrasalmidae Серебристый метиннис – Metynnis argenteus Silver dollar Yaroslavl 10 Обыкновенный метиннис – Metynnis schreitmuelleri (hypsauchen) Plainsilver dollar Nikolaev 4; Novosibirsk 100; Kharkov 20 Пятнистый метиннис – Metynnis maculatus Spotted metynnis Novosibirsk 50 Пиранья Наттерера – Serrasalmus nattereri Red piranha Novosibirsk 80; Kharkov 30 4 Сем. Харацидовые – Characidae Красноплавничный афиохаракс – Aphyocharax anisitsi (rubripinnis) Bloodfin tetra Киев 5; Perm 10 Парагвайский афиохаракс – Aphyocharax paraquayensis Whitespot tetra Perm 11 Рубиновый афиохаракс Рэтбина – Aphyocharax rathbuni Redflank bloodfin Perm 10 Эквадорская тетра – Astyanax sp. Tetra Perm 17 Слепая рыбка – Astyanax fasciatus mexicanus (Anoptichthys jordani) Mexican tetra Kharkov 10 Рублик-монетка – Ctenobrycon spilurus (+ С. spilurusvar. albino) Silver tetra Kharkov 20 Тернеция (Траурная тетра) – Gymnocorymbus -
![Wild Mammals of the Annapurna Conservation Area Cggk"0F{ ;+/If0f If]Qsf :Tgwf/L Jgohgt' Wild Mammals of the Annapurna Conservation Area - 2019](https://docslib.b-cdn.net/cover/7316/wild-mammals-of-the-annapurna-conservation-area-cggk-0f-if0f-if-qsf-tgwf-l-jgohgt-wild-mammals-of-the-annapurna-conservation-area-2019-127316.webp)
Wild Mammals of the Annapurna Conservation Area Cggk"0F{ ;+/If0f If]Qsf :Tgwf/L Jgohgt' Wild Mammals of the Annapurna Conservation Area - 2019
Wild Mammals of the Annapurna Conservation Area cGgk"0f{ ;+/If0f If]qsf :tgwf/L jGohGt' Wild Mammals of the Annapurna Conservation Area - 2019 ISBN 978-9937-8522-8-9978-9937-8522-8-9 9 789937 852289 National Trust for Nature Conservation Annapurna Conservation Area Project Khumaltar, Lalitpur, Nepal Hariyo Kharka, Pokhara, Kaski, Nepal National Trust for Nature Conservation P.O. Box: 3712, Kathmandu, Nepal P.O. Box: 183, Kaski, Nepal Tel: +977-1-5526571, 5526573, Fax: +977-1-5526570 Tel: +977-61-431102, 430802, Fax: +977-61-431203 Annapurna Conservation Area Project Email: [email protected] Email: [email protected] Website: www.ntnc.org.np Website: www.ntnc.org.np 2019 Wild Mammals of the Annapurna Conservation Area cGgk"0f{ ;+/If0f If]qsf :tgwf/L jGohGt' National Trust for Nature Conservation Annapurna Conservation Area Project 2019 Wild Mammals of the Annapurna Conservation Area cGgk"0f{ ;+/If0f If]qsf :tgwf/L jGohGt' Published by © NTNC-ACAP, 2019 All rights reserved Any reproduction in full or in part must mention the title and credit NTNC-ACAP. Reviewers Prof. Karan Bahadur Shah (Himalayan Nature), Dr. Naresh Subedi (NTNC, Khumaltar), Dr. Will Duckworth (IUCN) and Yadav Ghimirey (Friends of Nature, Nepal). Compilers Rishi Baral, Ashok Subedi and Shailendra Kumar Yadav Suggested Citation Baral R., Subedi A. & Yadav S.K. (Compilers), 2019. Wild Mammals of the Annapurna Conservation Area. National Trust for Nature Conservation, Annapurna Conservation Area Project, Pokhara, Nepal. First Edition : 700 Copies ISBN : 978-9937-8522-8-9 Front Cover : Yellow-bellied Weasel (Mustela kathiah), back cover: Orange- bellied Himalayan Squirrel (Dremomys lokriah). -

Checklist of the Mammals of Indonesia
CHECKLIST OF THE MAMMALS OF INDONESIA Scientific, English, Indonesia Name and Distribution Area Table in Indonesia Including CITES, IUCN and Indonesian Category for Conservation i ii CHECKLIST OF THE MAMMALS OF INDONESIA Scientific, English, Indonesia Name and Distribution Area Table in Indonesia Including CITES, IUCN and Indonesian Category for Conservation By Ibnu Maryanto Maharadatunkamsi Anang Setiawan Achmadi Sigit Wiantoro Eko Sulistyadi Masaaki Yoneda Agustinus Suyanto Jito Sugardjito RESEARCH CENTER FOR BIOLOGY INDONESIAN INSTITUTE OF SCIENCES (LIPI) iii © 2019 RESEARCH CENTER FOR BIOLOGY, INDONESIAN INSTITUTE OF SCIENCES (LIPI) Cataloging in Publication Data. CHECKLIST OF THE MAMMALS OF INDONESIA: Scientific, English, Indonesia Name and Distribution Area Table in Indonesia Including CITES, IUCN and Indonesian Category for Conservation/ Ibnu Maryanto, Maharadatunkamsi, Anang Setiawan Achmadi, Sigit Wiantoro, Eko Sulistyadi, Masaaki Yoneda, Agustinus Suyanto, & Jito Sugardjito. ix+ 66 pp; 21 x 29,7 cm ISBN: 978-979-579-108-9 1. Checklist of mammals 2. Indonesia Cover Desain : Eko Harsono Photo : I. Maryanto Third Edition : December 2019 Published by: RESEARCH CENTER FOR BIOLOGY, INDONESIAN INSTITUTE OF SCIENCES (LIPI). Jl Raya Jakarta-Bogor, Km 46, Cibinong, Bogor, Jawa Barat 16911 Telp: 021-87907604/87907636; Fax: 021-87907612 Email: [email protected] . iv PREFACE TO THIRD EDITION This book is a third edition of checklist of the Mammals of Indonesia. The new edition provides remarkable information in several ways compare to the first and second editions, the remarks column contain the abbreviation of the specific island distributions, synonym and specific location. Thus, in this edition we are also corrected the distribution of some species including some new additional species in accordance with the discovery of new species in Indonesia. -

Gut Analysis of Small Non-Volant Mammals of Mt. Makiling, Luzon Island, Philippines Anna Pauline O
Journal of Environmental Science and Management 17(2): 63-68 (December 2014) ISSN 0119-1144 Gut Analysis of Small Non-Volant Mammals of Mt. Makiling, Luzon Island, Philippines Anna Pauline O. de Guia1 and Ma. Niña Regina M. Quibod2 ABSTRACT Three non-native species (Rattus exulans, R. tanezumi and Mus musculus) of small non-volant mammals were recorded along various elevational gradients of Mount Makiling. Invertebrate remains and plant matter comprised the bulk of their diets based on the food items identifed. The identifed plant matter were leaves and seeds while invertebrates were easily identifable through body parts such as legs, head and antennae. Other contents identifed including vertebrate remains such as hair/fur, feathers and bones, plastics, rubber, stones, and intestinal worms were noted. Based on the calculated relative abundance of each food type, there is no signifcant difference in the diets of the three non-native rodent species. Preliminary results suggest that introduced rodents in Mt. Makiling have broad diets and there are no indications that their main diet includes native wildlife species. Traces of vertebrate remains, however, may indicate potential predation on wildlife species and further studies are needed to clarify this. Key words: rodents, gut analysis, endemic, non-native, elevational gradient INTRODUCTION The complexity of tropical mountain ecosystems endemic species (Rickart et al. 2007; Ong and Rickart 2008). have long provided haven for various Philippine wildlife R. exulans and R. tanezumi have been recorded at altitudes species. The elevational gradients provide various forest of 725 – 1450 masl on Mt. Isarog (Heaney et al. 1998). S. types while vertical stratifcation of trees offer habitat murinus, R. -

Norntates PUBLISHED by the AMERICAN MUSEUM of NATURAL HISTORY CENTRAL PARK WEST at 79TH STREET, NEW YORK, N.Y
AMERICAN MUSEUM Norntates PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK, N.Y. 10024 Number 3052, 19 pp., 9 figures, 1 table December 14, 1992 Sucking Lice (Insecta, Anoplura) from Indigenous Sulawesi Rodents: a New Species of Polyplax from a Montane Shrew Rat, and New Information About Polyplax wallacei and P. eropepli LANCE A. DURDEN' AND GUY G. MUSSER2 ABSTRACT Polyplax melasmothrixi, a new species of po- from Eropeplus canus from tropical upper mon- lyplacid sucking louse, is described from Melas- tane rain forest also in Central Sulawesi. Host and mothrix naso, a small-bodied shrew rat known habitat associations for these three species ofsuck- only from tropical upper montane rain forest in ing lice are discussed. Polyplax melasmothrixi and Central Sulawesi, Indonesia. The male ofPolyplax P. eropepli are both known only from montane wallacei is described from specimens collected from habitats in Central Sulawesi and both appear to Bunomys chrysocomus trapped in tropical lowland be host specific (to M. naso and E. canus, respec- evergreen rain forest in Central Sulawesi. A further tively). Contrastingly, P. wallacei parasitizes two specimen ofPolyplax eropepli, a taxon previously species ofBunomys in lowland forests and is known known only from the type series, is documented from North and Central Sulawesi. INTRODUCTION Melasmothrix naso, Bunomys chrysoco- Musser and Holden, 1991). The shrew rat, mus, and Eropeplus canus are three murine M. naso, and the large-bodied E. canus have rodents found only in forests on the Indo- been recorded only from montane rainforest nesian island of Sulawesi (Musser, 1987; formations in the mountainous central part I Assistant Professor and Assistant Curator, Institute of Arthropodology and Parasitology, Georgia Southern Uni- versity, Landrum Box 8056, Statesboro, Georgia 30460. -

TAXONOMIC STUDIES from RODENT OUTBREAK AREAS in the CHITTAGONG HILL TRACTS Nikhil
Bangladesh J. Zool. 46(2): 217-230, 2018 ISSN: 0304-9027 (print) 2408-8455 (online) NEW RECORDS OF RODENT SPECIES IN BANGLADESH: TAXONOMIC STUDIES FROM RODENT OUTBREAK AREAS IN THE CHITTAGONG HILL TRACTS Nikhil Chakma*, Noor Jahan Sarker, Steven Belmain1, Sohrab Uddin Sarker, Ken Aplin2 and Sontosh Kumar Sarker3 Department of Zoology, University of Dhaka, Dhaka-1000, Bangladesh Abstract: Rodents are regarded as crop pests, significant reservoirs and vectors for many zoonotic diseases around the world. Basic taxonomic information of rodents present in a locality can help understand which species are responsible as crop pest in that habitat. The phenomenon of the 50-year cycle of gregarious bamboo flowering and rodent outbreaks in the Chittagong Hill Tracts (CHT) of Bangladesh, rodents trapping were carried out in four habitats from March, 2009 to December, 2011 in Ruma upazila of Bandarban hill district. Variety of traps were used to capture small mammals. The captured species were measured and identified using taxonomical dichotomous keys and DNA bar-coding performed in Australia. A total of 14 different small mammalian species were captured of which nine belonging to the Muridae family, and one species each of Spalacidae, Sciuridae, Tupaiidae and Soricidae families. The dominant small mammal species captured were Rattus rattus (54.06%) followed by Mus musculus (26.39%), Rattus nitidus (10.98%), Suncus murinus (5.45%), Mus terricolor (1.09%), Mus cookii nagarum (0.97%), Cannomys badius (0.16%), Leopoldamys edwardsi (0.12%), Berylmys bowersi (0.12%), Vernaya fulva (0.08%), Rattus andamanensis (0.08%), Tupaia glis (0.04%) and Callosciurus pygerythrus (0.04%). -
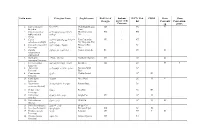
Red List of Endemic IUCN Red CITES Bern Bonn Georgia Species of the List Conventi Convention Caucasus on (CMS) 1
Latin name Georgian Name English name Red List of Endemic IUCN Red CITES Bern Bonn Georgia species of the list Conventi Convention Caucasus on (CMS) 1. Capra aegagrus niamori Wild Goat, Bezoar CR VU II Erxleben. Goat 2. Capra caucasica dasavleTkavkasiuri West Caucasian EN + EN Güldenstädt & jixvi Tur Pallas. 3. Capra aRmosavleTkavkasiuri East Caucasian VU + NT cylindricornis Blyth. jixvi Tur, Dagestan Tur 4. Capreolus capreolus evropuli Sveli European Roe LC Linnaeus. Deer 5. Gazella qurciki, jeirani Goitered Gazelle RE VU II subgutturosa Güldenstädt. 6. Rupicapra arCvi, fsiti Northern Chamois EN LC II rupicapra Linnaeus. 7. Cervus elaphus keTilSobili iremi Red Deer CR LC II I Linnaeus. 8. Sus scrofa gareuli Rori, taxi Eurasian Wild LC Linnaeus. Boar 9. Canis aureus tura Golden Jackal LC III Linnaeus. 10. Canis lupus mgeli Grey Wolf LC II II Linnaeus. 11. Nyctereutes enotisebri ZaRli Racoon Dog LC procyonoides Gray. 12. Vulpes vulpes mela Red Fox LC III Linnaeus. 13. Felis chaus lelianis kata Jungle Cat VU LC II Schreber. 14. Felis silvestris tyis kata Wild Cat LC II II Shreber. 15. Felis libyca Forster. velis kata Steppe Cat 16. Lynx lynx Linnaeus. focxveri Eurasian Lynx CR LC II 17. Panthera pardus jiqi Leopard CR NT I II Linnaeus. 18. Hyaena hyaena afTari Striped Hyaena CR NT Linnaeus. 19. Lutra lutra wavi Eurasian Otter, VU NT I II Linnaeus. Common Otter 20. Martes foina kldis kverna Stone Marten, LC III Erxleben. Beech Marten 21. Martes martes tyis kverna European Pine LC Linnaeus. Marten 22. Meles meles maCvi Eurasian Badger LC Linnaeus. 23. Mustela lutreola waula European Mink EN II Linnaeus. -

Quaternary Murid Rodents of Timor Part I: New Material of Coryphomys Buehleri Schaub, 1937, and Description of a Second Species of the Genus
QUATERNARY MURID RODENTS OF TIMOR PART I: NEW MATERIAL OF CORYPHOMYS BUEHLERI SCHAUB, 1937, AND DESCRIPTION OF A SECOND SPECIES OF THE GENUS K. P. APLIN Australian National Wildlife Collection, CSIRO Division of Sustainable Ecosystems, Canberra and Division of Vertebrate Zoology (Mammalogy) American Museum of Natural History ([email protected]) K. M. HELGEN Department of Vertebrate Zoology National Museum of Natural History Smithsonian Institution, Washington and Division of Vertebrate Zoology (Mammalogy) American Museum of Natural History ([email protected]) BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY Number 341, 80 pp., 21 figures, 4 tables Issued July 21, 2010 Copyright E American Museum of Natural History 2010 ISSN 0003-0090 CONTENTS Abstract.......................................................... 3 Introduction . ...................................................... 3 The environmental context ........................................... 5 Materialsandmethods.............................................. 7 Systematics....................................................... 11 Coryphomys Schaub, 1937 ........................................... 11 Coryphomys buehleri Schaub, 1937 . ................................... 12 Extended description of Coryphomys buehleri............................ 12 Coryphomys musseri, sp.nov.......................................... 25 Description.................................................... 26 Coryphomys, sp.indet.............................................. 34 Discussion . .................................................... -

Morphological Comparison of Ryukyu Mouse Mus Caroli
Zoological Studies 42(2): 258-267 (2003) Morphological Comparison of Ryukyu Mouse Mus caroli (Rodentia: Muridae) Populations from Okinawajima and Taiwan Masaharu Motokawa1,*, Liang-Kong Lin2 and Junko Motokawa3 1The Kyoto University Museum, Kyoto 606-8501, Japan 2Laboratory of Wildlife Ecology, Department of Biology, Tunghai University, Taichung, Taiwan 407, R.O.C. 3Department of Zoology, Graduate School of Science, Kyoto University, Kyoto 606-8502, Japan (Accepted January 14, 2003) Masaharu Motokawa, Liang-Kong Lin and Junko Motokawa (2003) Morphological comparison of Ryukyu mouse Mus caroli (Rodentia: Muridae) populations from Okinawajima and Taiwan. Zoological Studies 42(2): 258-267. We performed univariate, bivariate, and multivariate analyses of 4 external and 17 cranial morphome- tric characters in the Ryukyu mouse, Mus caroli Bonhote, 1902 (Mammalia: Rodentia: Muridae), using 177 specimens collected from Okinawajima in the central Ryukyus and from Taiwan. There were clear morphologi- cal differences between populations from Okinawajima and Taiwan. The univariate and bivariate analyses indi- cated that the Okinawajima population differs from the Taiwan population by a shorter tail, smaller ear and audi- tory bulla, less robust incisors, larger molar row, narrower cranium in the orbital region, and longer postpalatal region. Principal component and canonical discriminant analyses based on cranial variables also suggested morphological divergence between the 2 populations. http://www.sinica.edu.tw/zool/zoolstud/42.2/258.pdf Key words: Mus caroli, M. formosanus, Taxonomy, Morphometric analyses, Allometry. The Ryukyu mouse, Mus caroli, is distrib- ognize it as a valid species (e.g., Lin and Lin uted in the Ryukyu Archipelago (Japan), Taiwan, 1983). Hainan, and southern China to the Malay Another name included in M. -

Report on Biodiversity and Tropical Forests in Indonesia
Report on Biodiversity and Tropical Forests in Indonesia Submitted in accordance with Foreign Assistance Act Sections 118/119 February 20, 2004 Prepared for USAID/Indonesia Jl. Medan Merdeka Selatan No. 3-5 Jakarta 10110 Indonesia Prepared by Steve Rhee, M.E.Sc. Darrell Kitchener, Ph.D. Tim Brown, Ph.D. Reed Merrill, M.Sc. Russ Dilts, Ph.D. Stacey Tighe, Ph.D. Table of Contents Table of Contents............................................................................................................................. i List of Tables .................................................................................................................................. v List of Figures............................................................................................................................... vii Acronyms....................................................................................................................................... ix Executive Summary.................................................................................................................... xvii 1. Introduction............................................................................................................................1- 1 2. Legislative and Institutional Structure Affecting Biological Resources...............................2 - 1 2.1 Government of Indonesia................................................................................................2 - 2 2.1.1 Legislative Basis for Protection and Management of Biodiversity and -

Red List of Bangladesh Volume 2: Mammals
Red List of Bangladesh Volume 2: Mammals Lead Assessor Mohammed Mostafa Feeroz Technical Reviewer Md. Kamrul Hasan Chief Technical Reviewer Mohammad Ali Reza Khan Technical Assistants Selina Sultana Md. Ahsanul Islam Farzana Islam Tanvir Ahmed Shovon GIS Analyst Sanjoy Roy Technical Coordinator Mohammad Shahad Mahabub Chowdhury IUCN, International Union for Conservation of Nature Bangladesh Country Office 2015 i The designation of geographical entitles in this book and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN, International Union for Conservation of Nature concerning the legal status of any country, territory, administration, or concerning the delimitation of its frontiers or boundaries. The biodiversity database and views expressed in this publication are not necessarily reflect those of IUCN, Bangladesh Forest Department and The World Bank. This publication has been made possible because of the funding received from The World Bank through Bangladesh Forest Department to implement the subproject entitled ‘Updating Species Red List of Bangladesh’ under the ‘Strengthening Regional Cooperation for Wildlife Protection (SRCWP)’ Project. Published by: IUCN Bangladesh Country Office Copyright: © 2015 Bangladesh Forest Department and IUCN, International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holders, provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holders. Citation: Of this volume IUCN Bangladesh. 2015. Red List of Bangladesh Volume 2: Mammals. IUCN, International Union for Conservation of Nature, Bangladesh Country Office, Dhaka, Bangladesh, pp. -

Utility of a High-Resolution Mouse Single Nucleotide Polymorphism
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.29.318071; this version posted September 29, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Mouse single nucleotide polymorphic targets for cross hybridization in rodents 1 2 3 4 Utility of a high-resolution mouse single nucleotide polymorphism microarray assessed for 5 rodent comparative genomics 6 7 8 9 Short title: Mouse single nucleotide polymorphic targets for cross hybridization in rodents 10 11 12 13 Rachel D. Kelly, Maja Milojevic, Freda Qi, Kathleen A. Hill* 14 15 16 17 1Department of Biology, The University of Western Ontario, London, Ontario, Canada 18 19 20 * Corresponding author 21 22 Email: [email protected] (KH) 23 24 25 26 27 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.09.29.318071; this version posted September 29, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Mouse single nucleotide polymorphic targets for cross hybridization in rodents 28 Abstract 29 In the study of genetic diversity in non-model species there is a notable lack of the low-cost, high 30 resolution tools that are readily available for model organisms. Genotyping microarray 31 technology for model organisms is well-developed, affordable, and potentially adaptable for 32 cross-species hybridization.