Divergent Evolution in Antiherbivore Defences Within Species Complexes at a Single Amazonian Site
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution Activity, Grade 11
Evolution Activity, Grade 11 Evolution Activities for Grade 11 Students at the Toronto Zoo 1 Evolution Activity, Grade 11 Table of Contents Pre-Zoo Activity 3-8 • Think, Pair, Share – Animals in Society and Role of Zoos 3-5 o Description 3 o Materials 3 o Four Corners Activity 6 o Background Information 7-8 Zoo Activity 9-19 • Teacher’s Notes 9-13 o General Information, Curriculum expectations, 9-10 materials, procedure o Evaluation Rubrics 11-12 o Glossary 13 • Student Assignment 14-19 o Part 1 – Mission Preparation at the Zoo 15-16 (Observation Sheets) o Part 2 – Scientific Notes 17 o Part 3 – Documentation: The Story 18 o Appendix – Animal signs 19 Evaluation 20 2 Evolution Activity, Grade 11 Suggested Pre-zoo activity Time needed : 35 minutes (or more) Type of activity : pair-share, small-group (approximately 2-3 students) Objective : encourage students to think about and evaluate the roles of animals in our society and the purposes of zoos along with their own attitudes or stands toward zoos Materials needed : a set of 8-16 statements and a mode of ranking (either above the line-below the line or diamond style ranking system) Special note : In order to manage time, teacher can chose to use any number of the statements as long as the 4 core statements listed bellow are included. Task : students work together to rank the statements about the treatment of animals. They should work together and try to negotiate a consensus, but if this is impossible they can either leave out the particular statement or write down a few lines in their notes as to why they would place them in a different category. -

The Genetic Causes of Convergent Evolution
Nature Reviews Genetics | AOP, published online 9 October 2013; doi:10.1038/nrg3483 REVIEWS The genetic causes of convergent evolution David L. Stern Abstract | The evolution of phenotypic similarities between species, known as convergence, illustrates that populations can respond predictably to ecological challenges. Convergence often results from similar genetic changes, which can emerge in two ways: the evolution of similar or identical mutations in independent lineages, which is termed parallel evolution; and the evolution in independent lineages of alleles that are shared among populations, which I call collateral genetic evolution. Evidence for parallel and collateral evolution has been found in many taxa, and an emerging hypothesis is that they result from the fact that mutations in some genetic targets minimize pleiotropic effects while simultaneously maximizing adaptation. If this proves correct, then the molecular changes underlying adaptation might be more predictable than has been appreciated previously. (FIG. 1) Fitness Different species often evolve similar solutions to envi introgression . It is worth distinguishing between The potential evolutionary ronmental challenges. Insects, birds and bats evo these scenarios because each provides evidence for a dif success of a genotype, defined lved wings, and octopi, vertebrates and spiders ferent evolutionary path3. The first case, the independent as the reproductive success or evolved focusing eyes. Phenotypic convergence provides origin and spread of mutations, has been called parallel the proportion of genes that an individual leaves in the gene compelling evidence that ecological circumstances can genetic evolution. I suggest that the evolution of alleles 1,2 pool of the next generation in a select for similar evolutionary solutions . -

The Evolution of Mammalian Aging
Experimental Gerontology 37 &2002) 769±775 www.elsevier.com/locate/expgero The evolution of mammalian aging JoaÄo Pedro de MagalhaÄes*, Olivier Toussaint Department of Biology, Unit of Cellular Biochemistry and Biology, University of Namur FUNDP), Rue de Bruxelles 61, 5000 Namur, Belgium Received 18 December 2001; received in revised form 15 January 2002; accepted 18 January 2002 Abstract The incidence of aging is different between mammals and their closer ancestors &e.g. reptiles and amphibians). While all studied mammals express a well-de®ned aging phenotype, many amphibians and reptiles fail to show signs of aging. In addition, mammalian species show great similarities in their aging phenotype, suggesting that a common origin might be at work. The proposed hypothesis is that mammalian aging evolved together with the ancestry of modern mammals. In turn, this suggests that the fundamental cause of human aging is common to most, if not all, mammals and might be a unique phenom- enon. Experimental procedures capable of testing these theories and how to map the causes of mammalian and thus, human aging, are predicted. q 2002 Elsevier Science Inc. All rights reserved. Keywords: Aging; Senescence; Evolution; Mammals; Reptiles; Birds; Longevity 1. Introduction life span to Marion's tortoise capable of living over a century in captivity. Studies conducted in frogs failed Aging affects all studied mammalian species: from to indicate any increase in mortality both in the wild mice living no more than a mere half-a-dozen years to &Plytycz et al., 1995) and in captivity &Brocas and humans capable of living over 120years &Comfort, VerzaÁr, 1961). -

What Is Natural Selection?
This week (June 8th – 12th) we are reviewing evolution. 1. Review the lecture on evolution 2. Watch the evidence of evolution video: https://www.khanacademy.org/science/high-school-biology/hs-evolution/hs- evidence-of-evolution/v/evidence-for-evolution 3. Watch the Types of Natural Selection video https://youtu.be/64JUJdZdDQo 4. Complete the Evolution Review Activity 5. Complete the Natural Selection Reading and Questions I am grading you based on the rubric below. If you get below a 70% I will allow you to redo the assignment. Evidence of Evolution & the Week 5 Remote Learning Mechanisms of Biodiversity Natural Selection The total variety of all the organisms in the biosphere= Biodiversity Where did all these different organisms come from? How are they related? Some organisms in a population are less likely to survive. Over time, natural selection results in changes in the inherited characteristics of a population. WHAT IS DARWIN’S THEORY? These changes increase a species’ fitness in its environment. Any inherited characteristic Ability of an individual to that increases an organism’s survive and reproduce in its chance of survival= specific environment= FITNESS ADAPTATION WHAT IS DARWIN’S THEORY? STRUGGLE FOR EXISTENCE: means that members of each species must compete for food, space, and other resources. SURVIVAL OF THE FITTEST: organisms which are better adapted to the environment will survive and reproduce, passing on their genes. DESCENT WITH MODIFICATION: suggests that each species has decended, with changes, from other species over time. • This idea suggests that all living species are related to each other, and that all species, living and extinct,share a common ancestor. -

Evolution of Ant-Mimicking Beetles Edcator Materials
Evolution of Ant-Mimicking Beetles Data Point Educator Materials HOW TO USE THIS RESOURCE Show the following figures and caption to your students. The accompanying Student Handout provides space below the caption for Observations, Notes, and Questions and space next to the “Background Information” for Big Ideas, Notes, and Questions. The “Interpreting the Figures” and “Discussion Questions” sections provide additional information and suggested questions that you can use to prompt student thinking, increase engagement, or guide a class discussion about the characteristics of the figures and what they show. Figure 1: Figure 2: Caption: Figure 1 shows one species of ant-mimicking rove beetle (front) next to the ant species (back) that it mimics. The top half of Figure 2 shows more species of rove beetles from the same subfamily, Aleocharinae. “Free- living” beetles (example shown on the left) have a generalized beetle body type. “Army ant social parasite” beetles (examples shown on the right) have a specialized ant-mimicking (also called “myrmecoid”) body type. The bottom half of Figure 2 shows the evolutionary relationships among multiple Aleocharinae lineages that are either generalized (black) or ant-mimicking (orange). The labeled circle indicates the most recent common ancestor of all the ant-mimicking lineages. Phylogeny Published November 2019 www.BioInteractive.org Page 1 of 3 Data Point Evolution of Ant-Mimicking Beetles Educator Materials BACKGROUND INFORMATION Rove beetles are a large family of beetle species found all over the world. Although most rove beetles live freely on their own, many species in the subfamily Aleocharinae live closely with another type of insect: army ants. -

Evolutionary Origins for Ecological Patterns in Space PERSPECTIVE Mark C
PERSPECTIVE Evolutionary origins for ecological patterns in space PERSPECTIVE Mark C. Urbana,b,1, Sharon Y. Straussc, Fanie Pelletierd, Eric P. Palkovacse, Mathew A. Leiboldf, Andrew P. Hendryg,h, Luc De Meesteri,j,k, Stephanie M. Carlsonl, Amy L. Angertm, and Sean T. Gierya,n Edited by Simon A. Levin, Princeton University, Princeton, NJ, and approved June 5, 2020 (received for review January 24, 2020) Historically, many biologists assumed that evolution and ecology acted independently because evolution occurred over distances too great to influence most ecological patterns. Today, evidence indicates that evolution can operate over a range of spatial scales, including fine spatial scales. Thus, evolutionary divergence across space might frequently interact with the mechanisms that also determine spatial ecological patterns. Here, we synthesize insights from 500 eco-evolutionary studies and develop a predictive framework that seeks to understand whether and when evolution amplifies, dampens, or creates ecological patterns. We demonstrate that local adaptation can alter everything from spatial variation in population abundances to ecosystem properties. We uncover 14 mechanisms that can mediate the outcome of evolution on spatial ecological patterns. Sometimes, evolution amplifies environmental variation, especially when selection enhances resource uptake or patch selection. The local evolution of foundation or keystone species can create ecological patterns where none existed originally. However, most often, we find that evolution dampens existing environmental gradients, because local adaptation evens out fitness across environments and thus counteracts the variation in associated ecological patterns. Consequently, evolution generally smooths out the underlying heterogeneity in nature, making the world appear less ragged than it would be in the absence of evolution. -
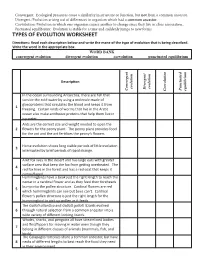
TYPES of EVOLUTION WORKSHEET Directions: Read Each Description Below and Write the Mane of the Type of Evolution That Is Being Described
Convergent: Ecological pressures cause a similarity in structure or function, but not from a common ancestor. Divergent: Evolution arising out of differences in organism which had a common ancestor. Coevolution: Evolution in which one organism causes another to change since they live in close association.. Pnctuated equilibrium: Evolution is stable for a time and suddenly jumps to new forms TYPES OF EVOLUTION WORKSHEET Directions: Read each description below and write the mane of the type of evolution that is being described. Write the word in the appropriate box. WORD BANK convergent evolution divergent evolution coevolution punctuated equilibrium Description evolution evolution Divergent Punctuated Convergent equilibrium Coevolution In the ocean surrounding Antarctica, there are fish that survive the cold water by using a molecule made of glycoproteins that circulates the blood and keeps it from 1 freezing. Certain kinds of worms that live in the Arctic ocean also make antifreeze proteins that help them live in icy water. Ants are the correct size and weight needed to open the 2 flowers for the peony plant. The peony plant provides food for the ant and the ant fertilizes the peony’s flowers Horse evolution shows long stable periods of little evolution 3 interrupted by brief periods of rapid change. A kit fox lives in the desert and has large ears with greater surface area that keep the fox from getting overheated. The 4 red fox lives in the forest and has a red coat that keeps it camouflaged. Hummingbirds have a beak just the right length to reach the nectar in a cardinal flower and as they feed their foreheads bump into the pollen structure. -

The Evolution of Mimicry
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Lake Forest College Publications Eukaryon Volume 4 Article 24 3-16-2008 The volutE ion of Mimicry Ethan Helm Lake Forest College Follow this and additional works at: http://publications.lakeforest.edu/eukaryon Part of the Animal Sciences Commons, Biodiversity Commons, and the Evolution Commons Disclaimer: Eukaryon is published by students at Lake Forest College, who are solely responsible for its content. The views expressed in Eukaryon do not necessarily reflect those of the College. Articles published within Eukaryon should not be cited in bibliographies. Material contained herein should be treated as personal communication and should be cited as such only with the consent of the author. This Review Article is brought to you for free and open access by the Student Publications at Lake Forest College Publications. It has been accepted for inclusion in Eukaryon by an authorized editor of Lake Forest College Publications. For more information, please contact [email protected]. Eukaryon, Vol. 4, March 2008, Lake Forest College ____________________________Review Article The Evolution of Mimicry Ethan Helm* deduced. This review is a comprehensive analysis of Department of Biology primary literature focusing on addressing these Lake Forest College evolutionary issues, while also evaluating the two-step Lake Forest, Illinois 60045 hypothesis and the gradual hypothesis of Müllerian mimicry. Introduction Mathematical Modeling of Mimicry Cryptic species have evolved camouflage, which enhances survival by decreasing their visibility and thus Mathematical and computerized modeling systems are protecting them from would-be predators. Conversely, commonly used to mimic the evolution of mimicry. -

Evolution Lesson 8.4: Macroevolution and the Origin of Species Lesson
Chapter 8: Evolution Lesson 8.4: Macroevolution and the Origin of Species Fast or slow? Which is better, the direct route or the scenic route? Each has its advantages, depending on the situation. And that describes evolution. It can be fast or slow, depending on the situation. Lesson Objectives • Describe two ways that new species may originate. • Differentiate between allopatric, peripatric, parapatric, and sympatric speciation. • Identify the different patterns of macroevolution • Distinguish between gradualism and punctuated equilibrium. • Understand the concept of extinction and how it affects evolution. Vocabulary • allopatric speciation • behavioral isolation • coevolution • convergent evolution • divergent evolution • geographic isolation • gradualism • parapatric speciation • peripatric speciation • punctuated equilibrium • speciation • sympatric speciation • temporal isolation Introduction Macroevolution is evolution over geologic time above the level of the species. One of the main topics in macroevolution is how new species arise. The process by which a new species evolves is called speciation. How does speciation occur? How does one species evolve into two or more new species? 275 Origin of Species To understand how a new species forms, it’s important to review what a species is. A species is a group of organisms that can breed and produce fertile offspring together in nature. For a new species to arise, some members of a species must become reproductively isolated from the rest of the species. This means they can no longer interbreed with other members of the species. How does this happen? Usually they become geographically isolated first. The macroevolution of a species happens as a result of speciation. Speciation is the branching off of individuals from the species they originally were. -

Patterns, Mechanisms and Genetics of Speciation in Reptiles and Amphibians
G C A T T A C G G C A T genes Review Patterns, Mechanisms and Genetics of Speciation in Reptiles and Amphibians Katharina C. Wollenberg Valero 1,* , Jonathon C. Marshall 2, Elizabeth Bastiaans 3, Adalgisa Caccone 4, Arley Camargo 5 , Mariana Morando 6, Matthew L. Niemiller 7, Maciej Pabijan 8 , Michael A. Russello 9, Barry Sinervo 10, Fernanda P. Werneck 11 , 12, 13 14 Jack W. Sites Jr. y, John J. Wiens and Sebastian Steinfartz 1 Department of Biological and Marine Sciences, University of Hull, Cottingham Road, Hull HU6 7RX, UK 2 Department of Zoology, Weber State University, 1415 Edvalson Street, Dept. 2505, Ogden, UT 84401, USA 3 Department of Biology, State University of New York, College at Oneonta, Oneonta, NY 13820, USA 4 Department of Ecology and Evolutionary Biology, Yale University, New Haven, CT 06520, USA 5 Centro Universitario de Rivera, Universidad de la República, Ituzaingó 667, Rivera 40000, Uruguay 6 Instituto Patagónico para el Estudio de los Ecosistemas Continentales (IPEEC, CENPAT-CONICET) Bv. Brown 2915, Puerto Madryn U9120ACD, Argentina 7 Department of Biological Sciences, The University of Alabama in Huntsville, Huntsville, AL 35899, USA 8 Department of Comparative Anatomy, Institute of Zoology and Biomedical Research, Jagiellonian University, ul. Gronostajowa 9, 30-387 Kraków, Poland 9 Department of Biology, University of British Columbia, Okanagan Campus, 3247 University Way, Kelowna, BC V1V 1V7, Canada 10 Department of Ecology and Evolutionary Biology, University of California, Santa Cruz, Coastal Biology Building, -

Divergent Evolution and Evolution by the Birth-And-Death Process in the Immunoglobulin VH Gene Family
Divergent Evolution and Evolution by the Birth-and-Death Process in the Immunoglobulin VH Gene Family Tatsuya Ota and Masatoshi Nei Department of Biology and Institute of Molecular Evolutionary Genetics, The Pennsylvania State University Immunoglobulin diversity is generated primarily by the heavy- and light-chain variable-region gene families. To understand the pattern of long-term evolution of the heavy-chain variable-region (V,) gene family, which is composed of a large number of member genes, the evolutionary relationships of representative Vn genes from diverse organisms of vertebrates were studied by constructing a phylogenetic tree. This tree indicates that the vertebrate Vn genes can be classified into group A, B, C, D, and E genes. All Vn genes from cartilaginous fishes such as sharks and skates form a monophyletic group and belong to group E, whereas group D consists of bony- fish Vn genes. By contrast, group C includes not only some fish genes but also amphibian, reptile, bird, and mammalian genes. Group A and B genes were composed of the genes from mammals and amphibians. The phylogenetic analysis also suggests that mammalian Vu genes are classified into three clusters-i.e., mammalian clans I, II, and III-and that these clans have coexisted in the genome for >400 Myr. To study the short-term evolution of Vn genes, the phylogenetic analysis of human group A (clan I) and C (clan III) genes was also conducted. The results obtained show that Vn pseudogenes have evolved much faster than functional genes and that they have branched off from various functional Vu genes. -

9Th Grade Biology: Population Genetics and Speciation April 20 – April 23 Time Allotment: 40 Minutes Per Day
9th Grade Biology: Population Genetics and Speciation April 20 – April 23 Time Allotment: 40 minutes per day Student Name: ________________________________ Period: ______ Teacher Name: Ms. Carstens 9th Biology – Population Genetics and Speciation April 20 – April 23 Student Name: ____________________________________ Packet Overview Date Objective(s) Page # Monday, April 20 1. Distinguish between coevolution, convergent evolution, 2 and divergent evolution. 2. Explain the significance of the gene pool in relation to evolution. Tuesday, April 21 1. Explain the Hardy-Weinberg genetic equilibrium. 7 2. Identify disruptions in the Hardy-Weinberg genetic equilibrium that may result in evolution of a species. Wednesday, April 22 1. Compare the effects of sexual selection, stabilizing 11 selection, disruptive selection, and directional selection. Thursday, April 23 1. Describe how isolation can lead to speciation. 15 2. Compare gradualism with punctuated equilibrium. Friday, April 24 NO CLASS Additional Notes: Hi all! I hope you are doing well and adjusting to our new learning avenues! Remember, I am here for you—through email ([email protected]) and also my Zoom office hours. Zoom sessions are intended for the purpose of asking questions, clarifying instructions, and seeking more information on the content topics for the week. If you need to attend, please join the session that corresponds with your class schedule. The times are listed below: • 1st Period – Mondays, Wednesdays from 10:00-10:50 am • 3rd Period – Mondays, Wednesdays from 1:00-1:50 pm • 4th Period – Tuesdays, Thursdays from 10:00-10:50 am • 6th Period – Tuesdays, Thursdays from 1:00-1:50 pm Per usual, your weekly minor assessment is found on pgs.