Download (6MB)
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

13914444D46c0aa91d02e31218
2 Breeding of wild and some domestic animals at regional zoological institutions in 2013 3 РЫБЫ P I S C E S ВОББЕЛОНГООБРАЗНЫЕ ORECTOLOBIFORMES Сем. Азиатские кошачьи акулы (Бамбуковые акулы) – Hemiscyllidae Коричневополосая бамбуковая акула – Chiloscyllium punctatum Brownbanded bambooshark IUCN (NT) Sevastopol 20 ХВОСТОКОЛООБРАЗНЫЕ DASYATIFORMES Сем. Речные хвостоколы – Potamotrygonidae Глазчатый хвостокол (Моторо) – Potamotrygon motoro IUCN (DD) Ocellate river stingray Sevastopol - ? КАРПООБРАЗНЫЕ CYPRINIFORMES Сем. Цитариновые – Citharinidae Серебристый дистиход – Distichodusaffinis (noboli) Silver distichodus Novosibirsk 40 Сем. Пираньевые – Serrasalmidae Серебристый метиннис – Metynnis argenteus Silver dollar Yaroslavl 10 Обыкновенный метиннис – Metynnis schreitmuelleri (hypsauchen) Plainsilver dollar Nikolaev 4; Novosibirsk 100; Kharkov 20 Пятнистый метиннис – Metynnis maculatus Spotted metynnis Novosibirsk 50 Пиранья Наттерера – Serrasalmus nattereri Red piranha Novosibirsk 80; Kharkov 30 4 Сем. Харацидовые – Characidae Красноплавничный афиохаракс – Aphyocharax anisitsi (rubripinnis) Bloodfin tetra Киев 5; Perm 10 Парагвайский афиохаракс – Aphyocharax paraquayensis Whitespot tetra Perm 11 Рубиновый афиохаракс Рэтбина – Aphyocharax rathbuni Redflank bloodfin Perm 10 Эквадорская тетра – Astyanax sp. Tetra Perm 17 Слепая рыбка – Astyanax fasciatus mexicanus (Anoptichthys jordani) Mexican tetra Kharkov 10 Рублик-монетка – Ctenobrycon spilurus (+ С. spilurusvar. albino) Silver tetra Kharkov 20 Тернеция (Траурная тетра) – Gymnocorymbus -

Evolution Activity, Grade 11
Evolution Activity, Grade 11 Evolution Activities for Grade 11 Students at the Toronto Zoo 1 Evolution Activity, Grade 11 Table of Contents Pre-Zoo Activity 3-8 • Think, Pair, Share – Animals in Society and Role of Zoos 3-5 o Description 3 o Materials 3 o Four Corners Activity 6 o Background Information 7-8 Zoo Activity 9-19 • Teacher’s Notes 9-13 o General Information, Curriculum expectations, 9-10 materials, procedure o Evaluation Rubrics 11-12 o Glossary 13 • Student Assignment 14-19 o Part 1 – Mission Preparation at the Zoo 15-16 (Observation Sheets) o Part 2 – Scientific Notes 17 o Part 3 – Documentation: The Story 18 o Appendix – Animal signs 19 Evaluation 20 2 Evolution Activity, Grade 11 Suggested Pre-zoo activity Time needed : 35 minutes (or more) Type of activity : pair-share, small-group (approximately 2-3 students) Objective : encourage students to think about and evaluate the roles of animals in our society and the purposes of zoos along with their own attitudes or stands toward zoos Materials needed : a set of 8-16 statements and a mode of ranking (either above the line-below the line or diamond style ranking system) Special note : In order to manage time, teacher can chose to use any number of the statements as long as the 4 core statements listed bellow are included. Task : students work together to rank the statements about the treatment of animals. They should work together and try to negotiate a consensus, but if this is impossible they can either leave out the particular statement or write down a few lines in their notes as to why they would place them in a different category. -

Divergent Evolution in Antiherbivore Defences Within Species Complexes at a Single Amazonian Site
Journal of Ecology 2015, 103, 1107–1118 doi: 10.1111/1365-2745.12431 Divergent evolution in antiherbivore defences within species complexes at a single Amazonian site Marıa-Jose Endara1*, Alexander Weinhold1, James E. Cox2, Natasha L. Wiggins1, Phyllis D. Coley1,3 and Thomas A. Kursar1,3 1Department of Biology, The University of Utah, Salt Lake City, UT 84112-0840, USA; 2Health Sciences Center Core Research Facilities, School of Medicine, The University of Utah, Salt Lake City, UT 84112-0840, USA; and 3Smithsonian Tropical Research Institute, Balboa Box 0843-03092, Panama Summary 1. Classic theory in plant–insect interactions has linked herbivore pressure with diversification in plant species. We hypothesize that herbivores may exert divergent selection on defences, such that closely related plant species will be more different in defensive than in non-defensive traits. 2. We evaluated this hypothesis by investigating two clades of closely related plant species coexis- ting at a single site in the Peruvian Amazon: Inga capitata Desv. and Inga heterophylla Willd. spe- cies complexes. We compared how these lineages differ in the suite of chemical, biotic, phenological and developmental defences as compared to non-defensive traits that are related to habitat use and resource acquisition. We also collected insect herbivores feeding on the plants. 3. Our data show that sister lineages within both species complexes are more divergent in antiherbi- vore defences than in other non-defensive, functional traits. Moreover, the assemblages of herbivore communities are dissimilar between the populations of coexisting I. capitata lineages. 4. Synthesis. Our results are consistent with the idea that for the I. -

Clicking Caterpillars: Acoustic Aposematism in Antheraea Polyphemus and Other Bombycoidea Sarah G
993 The Journal of Experimental Biology 210, 993-1005 Published by The Company of Biologists 2007 doi:10.1242/jeb.001990 Clicking caterpillars: acoustic aposematism in Antheraea polyphemus and other Bombycoidea Sarah G. Brown1, George H. Boettner2 and Jayne E. Yack1,* 1Department of Biology, Carleton University, Ottawa, Ontario, K1S 5B6, Canada and 2Plant Soil and Insect Sciences, University of Massachusetts, Amherst, MA 01003, USA *Author for correspondence (e-mail: [email protected]) Accepted 21 November 2006 Summary Acoustic signals produced by caterpillars have been correlated sound production with attack, and an increase documented for over 100 years, but in the majority of in attack rate was positively correlated with the number of cases their significance is unknown. This study is the first signals produced. In addition, sound production typically to experimentally examine the phenomenon of audible preceded or accompanied defensive regurgitation. sound production in larval Lepidoptera, focusing on a Bioassays with invertebrates (ants) and vertebrates (mice) common silkmoth caterpillar, Antheraea polyphemus revealed that the regurgitant is deterrent to would-be (Saturniidae). Larvae produce airborne sounds, predators. Comparative evidence revealed that other resembling ‘clicks’, with their mandibles. Larvae typically Bombycoidea species, including Actias luna (Saturniidae) signal multiple times in quick succession, producing trains and Manduca sexta (Sphingidae), also produce airborne that last over 1·min and include 50–55 clicks. Individual sounds upon attack, and that these sounds precede clicks within a train are on average 24.7·ms in duration, regurgitation. The prevalence and adaptive significance of often consisting of multiple components. Clicks are audible warning sounds in caterpillars is discussed. -

A Survey on Sphingidae (Lepidoptera) Species of South Eastern Turkey
Cumhuriyet Science Journal e-ISSN: 2587-246X Cumhuriyet Sci. J., 41(1) (2020) 319-326 ISSN: 2587-2680 http://dx.doi.org/10.17776/csj.574903 A survey on sphingidae (lepidoptera) species of south eastern Turkey with new distributional records Erdem SEVEN 1 * 1 Department of Gastronomy and Culinary Arts, School of Tourism and Hotel Management, Batman University, 72060, Batman, Turkey. Abstract Article info History: This paper provides comments on the Sphingidae species of south eastern Turkey by the field Received:10.06.2019 surveys are conducted between in 2015-2017. A total of 15 species are determined as a result Accepted:20.12.2019 of the investigations from Batman, Diyarbakır and Mardin provinces. With this study, the Keywords: number of sphinx moths increased to 13 in Batman, 14 in Diyarbakır and 8 in Mardin. Among Fauna, them, 7 species for Batman, 4 species for Diyarbakır and 1 species for Mardin are new record. Hawk moths, For each species, original reference, type locality, material examined, distribution in the world New records, and in Turkey, and larval hostplants are given. Adults figures of Smerinthus kindermanni Sphingidae, Lederer, 1852; Marumba quercus ([Denis & Schiffermüller], 1775); Rethera komarovi Turkey. (Christoph, 1885); Macroglossum stellatarum (Linnaeus, 1758); Hyles euphorbiae (Linnaeus, 1758) and H. livornica (Esper, [1780]) are illustrated. 1. Introduction 18, 22-24]: Acherontia atropos (Linnaeus, 1758); Agrius convolvuli (Linnaeus, 1758); Akbesia davidi (Oberthür, 1884); Clarina kotschyi (Kollar, [1849]); C. The Sphingidae family classified in the Sphingoidea syriaca (Lederer, 1855); Daphnis nerii (Linnaeus, Superfamily and species of the family are generally 1758); Deilephila elpenor (Linnaeus, 1758); D. -
Macrogyrodactylus Polypteri Malberg on Polypterus Senegalus in the Sudan
Macrogyrodactylus polypteri Malberg on Polypterus senegalus in the Sudan Item Type article Authors Amirthalingam, C. Download date 26/09/2021 20:17:20 Link to Item http://hdl.handle.net/1834/32496 NOTES AND COMMENTS Macrogyrodactylus polypter£ Malberg on Polypterus senega/us in the Sudan POLYPTERUS is a genua of fishes which is best considered as descendent ()f the Palaeoniscid stock coming from Devonian times (400 million years ago). This genus, one of the' indigenous fishes of Africa, is of economic importance in some parts of the Sudan. In August 1962, small specimens of Polypterus senegalus Cuvier, ranging from 20 to 25 ems., collected from Jebel el Aulia on the While Nile, were introduced into a well aerated aquarium and fed on earthworms. During the first week the water was clear in the tank; nevertheless, because of the debris from the earthworms, the water was changed once or twice. The fishes appeared to be quite active and healthy and were swimming in mid-water or resting on the fl.oor of the aquarium. Occasionally they came up to the surface to take a gulp of air. In the course of the following week, the water-although changed as frequently as before-appeared to become turbid and viscous. At the end of that week, a few of the fishes were found to be lethargic, drifting with the dorsal finlets and a row or two of the dorsolateral scales exposed above the water level. On the 15th day some died. Post-mortem examination revealed that the dead fishes were heavily infected with a monogenetic trematode of the Gyrodactylid type which was later identified (by my colleague L. -

The Genetic Causes of Convergent Evolution
Nature Reviews Genetics | AOP, published online 9 October 2013; doi:10.1038/nrg3483 REVIEWS The genetic causes of convergent evolution David L. Stern Abstract | The evolution of phenotypic similarities between species, known as convergence, illustrates that populations can respond predictably to ecological challenges. Convergence often results from similar genetic changes, which can emerge in two ways: the evolution of similar or identical mutations in independent lineages, which is termed parallel evolution; and the evolution in independent lineages of alleles that are shared among populations, which I call collateral genetic evolution. Evidence for parallel and collateral evolution has been found in many taxa, and an emerging hypothesis is that they result from the fact that mutations in some genetic targets minimize pleiotropic effects while simultaneously maximizing adaptation. If this proves correct, then the molecular changes underlying adaptation might be more predictable than has been appreciated previously. (FIG. 1) Fitness Different species often evolve similar solutions to envi introgression . It is worth distinguishing between The potential evolutionary ronmental challenges. Insects, birds and bats evo these scenarios because each provides evidence for a dif success of a genotype, defined lved wings, and octopi, vertebrates and spiders ferent evolutionary path3. The first case, the independent as the reproductive success or evolved focusing eyes. Phenotypic convergence provides origin and spread of mutations, has been called parallel the proportion of genes that an individual leaves in the gene compelling evidence that ecological circumstances can genetic evolution. I suggest that the evolution of alleles 1,2 pool of the next generation in a select for similar evolutionary solutions . -

Growth and Reproductive Parameters of Polypterus Senegalus Cuvier 1829 in Eleiyele Lake
New York Science Journal 2016;9(11) http://www.sciencepub.net/newyork Growth and reproductive parameters of Polypterus senegalus Cuvier 1829 in Eleiyele Lake Adedolapo Abeke Ayoade and Juliet Avwesuruo Akponine Department of Zoology, University of Ibadan, Oyo State, Nigeria. *Corresponding author E-mail: [email protected] Phone Number: +234-8033855807 Abstract: The Senegal bichir, Polypterus senegalus Cuvier 1829 is of commercial importance as food and ornamental fish. This study describes the growth pattern and aspects of reproductive biology for the species in the Eleyele Lake, Nigeria. One hundred and twenty nine specimens were collected from October, 2010 to April, 2011. For each individual, the total length, standard length and body weight were measured also aspects of reproductive biology (gonadosomatic index, fecundity, egg diameter) were determined. All the LWRs showed strong correlations (r> 0.75, p>0.05). The b value obtained varies with body size and higher value was recorded for the smaller size group. The mean K for the combined sexes was 0.536 0.007. Absolute fecundity ranged between 622 (for specimen with TL = 16.4 cm; total weight = 21.61 g) and 2593 eggs (for specimen with TL = 27.7 cm; total weight = 120.62 g). The frequency polygons of the egg diameter suggest the species is a multiple spawner. [Adedolapo Abeke Ayoade and Juliet Avwesuruo Akponine. Growth and reproductive parameters of Polypterus senegalus Cuvier 1829 in Eleiyele Lake. N Y Sci J 2016;9(11):27-31]. ISSN 1554-0200 (print); ISSN 2375-723X (online). http://www.sciencepub.net/newyork. 5. doi:10.7537/marsnys091116.05. -
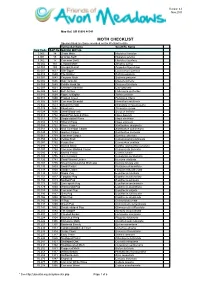
MOTH CHECKLIST Species Listed Are Those Recorded on the Wetland to Date
Version 4.0 Nov 2015 Map Ref: SO 95086 46541 MOTH CHECKLIST Species listed are those recorded on the Wetland to date. Vernacular Name Scientific Name New Code B&F No. MACRO MOTHS 3.005 14 Ghost Moth Hepialus humulae 3.001 15 Orange Swift Hepialus sylvina 3.002 17 Common Swift Hepialus lupulinus 50.002 161 Leopard Moth Zeuzera pyrina 54.008 169 Six-spot Burnet Zygaeba filipendulae 66.007 1637 Oak Eggar Lasiocampa quercus 66.010 1640 The Drinker Euthrix potatoria 68.001 1643 Emperor Moth Saturnia pavonia 65.002 1646 Oak Hook-tip Drepana binaria 65.005 1648 Pebble Hook-tip Drepana falcataria 65.007 1651 Chinese Character Cilix glaucata 65.009 1653 Buff Arches Habrosyne pyritoides 65.010 1654 Figure of Eighty Tethia ocularis 65.015 1660 Frosted Green Polyploca ridens 70.305 1669 Common Emerald Hermithea aestivaria 70.302 1673 Small Emerald Hemistola chrysoprasaria 70.029 1682 Blood-vein Timandra comae 70.024 1690 Small Blood-vein Scopula imitaria 70.013 1702 Small Fan-footed Wave Idaea biselata 70.011 1708 Single-dotted Wave Idaea dimidiata 70.016 1713 Riband Wave Idaea aversata 70.053 1722 Flame Carpet Xanthorhoe designata 70.051 1724 Red Twin-spot Carpet Xanthorhoe spadicearia 70.049 1728 Garden Carpet Xanthorhoe fluctuata 70.061 1738 Common Carpet Epirrhoe alternata 70.059 1742 Yellow Shell Camptogramma bilineata 70.087 1752 Purple Bar Cosmorhoe ocellata 70.093 1758 Barred Straw Eulithis (Gandaritis) pyraliata 70.097 1764 Common Marbled Carpet Chloroclysta truncata 70.085 1765 Barred Yellow Cidaria fulvata 70.100 1776 Green Carpet Colostygia pectinataria 70.126 1781 Small Waved Umber Horisme vitalbata 70.107 1795 November/Autumnal Moth agg Epirrita dilutata agg. -

Results of a Lepidopterological Expedition to North and Northwest Iran in Summer 2007 with New Records for Iran (Lepidoptera) (Plates 19-22)
Esperiana Band 16: 135-165 Schwanfeld, 06. Dezember 2011 ISBN 978-3-938249-01-7 Results of a lepidopterological expedition to North and Northwest Iran in summer 2007 with new records for Iran (Lepidoptera) (plates 19-22) Lutz LEHMANN † & Reza ZAHIRI Abstract: The results of a joint lepidopterological expedition to North and Northwest Iran, povinces of Tehran, Mazandaran, Guilan, Ardabil and Azerbaijan-e-Sharqi, from 20 July to 1 August are presented. More than 508 species of Macrolepidoptera (sensu SEITZ) could be recorded, among them Idaea sericeata (HÜBNER, 1813), Cinglis humifusaria EVERSMANN, 1837, Aplocera uniformata (URBAHN, 1971), Scotopteryx chenopodiata (LINNAEUS, 1758), Triphosa dubitata (LINANEUS, 1758), Abraxas grossulariata (LINNAEUS, 1758), Kemtrognophos ciscaucasica (RJABOV, 1964), Furcula danieli SCHINTLMEISTER, 1998, Eilema lurideola ([ZINCKEN], 1817), Zethes propinquus CHRISTOPH, 1885, Meganola togatulalis (HÜBNER, 1796), Meganola kolbi (DANIEL, 1935), Pseudluperina pozzii (CURÓ, 1883), Mythimna sicula scirpi (DUPONCHEL, 1836) and Noctua interposita (HÜBNER, 1790) new for the fauna of Iran. Additionally, the species of two light traps in the Elburs Mts., province Mazandaran, from the beginning of June, collected by A. PÜTZ, are listed. The genitalia of 16 species, typical habitats, and some living and spread specimens are figured. Zusammenfassung: Die Ergebnisse einer gemeinsamen lepidopterologischen Expedition in den Nord- und Nordwestiran, Provinzen Teheran, Mazandaran, Guilan, Ardabil and Azerbaijan-e-Sharqi, vom -

The Evolution of Mammalian Aging
Experimental Gerontology 37 &2002) 769±775 www.elsevier.com/locate/expgero The evolution of mammalian aging JoaÄo Pedro de MagalhaÄes*, Olivier Toussaint Department of Biology, Unit of Cellular Biochemistry and Biology, University of Namur FUNDP), Rue de Bruxelles 61, 5000 Namur, Belgium Received 18 December 2001; received in revised form 15 January 2002; accepted 18 January 2002 Abstract The incidence of aging is different between mammals and their closer ancestors &e.g. reptiles and amphibians). While all studied mammals express a well-de®ned aging phenotype, many amphibians and reptiles fail to show signs of aging. In addition, mammalian species show great similarities in their aging phenotype, suggesting that a common origin might be at work. The proposed hypothesis is that mammalian aging evolved together with the ancestry of modern mammals. In turn, this suggests that the fundamental cause of human aging is common to most, if not all, mammals and might be a unique phenom- enon. Experimental procedures capable of testing these theories and how to map the causes of mammalian and thus, human aging, are predicted. q 2002 Elsevier Science Inc. All rights reserved. Keywords: Aging; Senescence; Evolution; Mammals; Reptiles; Birds; Longevity 1. Introduction life span to Marion's tortoise capable of living over a century in captivity. Studies conducted in frogs failed Aging affects all studied mammalian species: from to indicate any increase in mortality both in the wild mice living no more than a mere half-a-dozen years to &Plytycz et al., 1995) and in captivity &Brocas and humans capable of living over 120years &Comfort, VerzaÁr, 1961). -

Bichir External Gills Arise Via Heterochronic Shift That Accelerates
RESEARCH ARTICLE Bichir external gills arise via heterochronic shift that accelerates hyoid arch development Jan Stundl1,2, Anna Pospisilova1, David Jandzik1,3, Peter Fabian1†, Barbora Dobiasova1‡, Martin Minarik1§, Brian D Metscher4, Vladimir Soukup1*, Robert Cerny1* 1Department of Zoology, Faculty of Science, Charles University in Prague, Prague, Czech Republic; 2National Museum, Prague, Czech Republic; 3Department of Zoology, Faculty of Natural Sciences, Comenius University in Bratislava, Bratislava, Slovakia; 4Department of Theoretical Biology, University of Vienna, Vienna, Austria *For correspondence: Abstract In most vertebrates, pharyngeal arches form in a stereotypic anterior-to-posterior [email protected] progression. To gain insight into the mechanisms underlying evolutionary changes in pharyngeal (VS); arch development, here we investigate embryos and larvae of bichirs. Bichirs represent the earliest [email protected] (RC) diverged living group of ray-finned fishes, and possess intriguing traits otherwise typical for lobe- Present address: †Eli and Edythe finned fishes such as ventral paired lungs and larval external gills. In bichir embryos, we find that Broad CIRM Center for the anteroposterior way of formation of cranial segments is modified by the unique acceleration of Regenerative Medicine and Stem the entire hyoid arch segment, with earlier and orchestrated development of the endodermal, Cell Research, University of mesodermal, and neural crest tissues. This major heterochronic shift in the anteroposterior Southern California, Los Angeles, developmental sequence enables early appearance of the external gills that represent key ‡ United States; The Prague breathing organs of bichir free-living embryos and early larvae. Bichirs thus stay as unique models Zoological Garden, Prague, for understanding developmental mechanisms facilitating increased breathing capacity.