Biosystems ISSN 2520-2529 (Online) Biosyst
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clicking Caterpillars: Acoustic Aposematism in Antheraea Polyphemus and Other Bombycoidea Sarah G
993 The Journal of Experimental Biology 210, 993-1005 Published by The Company of Biologists 2007 doi:10.1242/jeb.001990 Clicking caterpillars: acoustic aposematism in Antheraea polyphemus and other Bombycoidea Sarah G. Brown1, George H. Boettner2 and Jayne E. Yack1,* 1Department of Biology, Carleton University, Ottawa, Ontario, K1S 5B6, Canada and 2Plant Soil and Insect Sciences, University of Massachusetts, Amherst, MA 01003, USA *Author for correspondence (e-mail: [email protected]) Accepted 21 November 2006 Summary Acoustic signals produced by caterpillars have been correlated sound production with attack, and an increase documented for over 100 years, but in the majority of in attack rate was positively correlated with the number of cases their significance is unknown. This study is the first signals produced. In addition, sound production typically to experimentally examine the phenomenon of audible preceded or accompanied defensive regurgitation. sound production in larval Lepidoptera, focusing on a Bioassays with invertebrates (ants) and vertebrates (mice) common silkmoth caterpillar, Antheraea polyphemus revealed that the regurgitant is deterrent to would-be (Saturniidae). Larvae produce airborne sounds, predators. Comparative evidence revealed that other resembling ‘clicks’, with their mandibles. Larvae typically Bombycoidea species, including Actias luna (Saturniidae) signal multiple times in quick succession, producing trains and Manduca sexta (Sphingidae), also produce airborne that last over 1·min and include 50–55 clicks. Individual sounds upon attack, and that these sounds precede clicks within a train are on average 24.7·ms in duration, regurgitation. The prevalence and adaptive significance of often consisting of multiple components. Clicks are audible warning sounds in caterpillars is discussed. -

A Survey on Sphingidae (Lepidoptera) Species of South Eastern Turkey
Cumhuriyet Science Journal e-ISSN: 2587-246X Cumhuriyet Sci. J., 41(1) (2020) 319-326 ISSN: 2587-2680 http://dx.doi.org/10.17776/csj.574903 A survey on sphingidae (lepidoptera) species of south eastern Turkey with new distributional records Erdem SEVEN 1 * 1 Department of Gastronomy and Culinary Arts, School of Tourism and Hotel Management, Batman University, 72060, Batman, Turkey. Abstract Article info History: This paper provides comments on the Sphingidae species of south eastern Turkey by the field Received:10.06.2019 surveys are conducted between in 2015-2017. A total of 15 species are determined as a result Accepted:20.12.2019 of the investigations from Batman, Diyarbakır and Mardin provinces. With this study, the Keywords: number of sphinx moths increased to 13 in Batman, 14 in Diyarbakır and 8 in Mardin. Among Fauna, them, 7 species for Batman, 4 species for Diyarbakır and 1 species for Mardin are new record. Hawk moths, For each species, original reference, type locality, material examined, distribution in the world New records, and in Turkey, and larval hostplants are given. Adults figures of Smerinthus kindermanni Sphingidae, Lederer, 1852; Marumba quercus ([Denis & Schiffermüller], 1775); Rethera komarovi Turkey. (Christoph, 1885); Macroglossum stellatarum (Linnaeus, 1758); Hyles euphorbiae (Linnaeus, 1758) and H. livornica (Esper, [1780]) are illustrated. 1. Introduction 18, 22-24]: Acherontia atropos (Linnaeus, 1758); Agrius convolvuli (Linnaeus, 1758); Akbesia davidi (Oberthür, 1884); Clarina kotschyi (Kollar, [1849]); C. The Sphingidae family classified in the Sphingoidea syriaca (Lederer, 1855); Daphnis nerii (Linnaeus, Superfamily and species of the family are generally 1758); Deilephila elpenor (Linnaeus, 1758); D. -
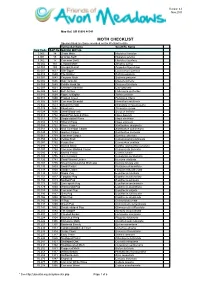
MOTH CHECKLIST Species Listed Are Those Recorded on the Wetland to Date
Version 4.0 Nov 2015 Map Ref: SO 95086 46541 MOTH CHECKLIST Species listed are those recorded on the Wetland to date. Vernacular Name Scientific Name New Code B&F No. MACRO MOTHS 3.005 14 Ghost Moth Hepialus humulae 3.001 15 Orange Swift Hepialus sylvina 3.002 17 Common Swift Hepialus lupulinus 50.002 161 Leopard Moth Zeuzera pyrina 54.008 169 Six-spot Burnet Zygaeba filipendulae 66.007 1637 Oak Eggar Lasiocampa quercus 66.010 1640 The Drinker Euthrix potatoria 68.001 1643 Emperor Moth Saturnia pavonia 65.002 1646 Oak Hook-tip Drepana binaria 65.005 1648 Pebble Hook-tip Drepana falcataria 65.007 1651 Chinese Character Cilix glaucata 65.009 1653 Buff Arches Habrosyne pyritoides 65.010 1654 Figure of Eighty Tethia ocularis 65.015 1660 Frosted Green Polyploca ridens 70.305 1669 Common Emerald Hermithea aestivaria 70.302 1673 Small Emerald Hemistola chrysoprasaria 70.029 1682 Blood-vein Timandra comae 70.024 1690 Small Blood-vein Scopula imitaria 70.013 1702 Small Fan-footed Wave Idaea biselata 70.011 1708 Single-dotted Wave Idaea dimidiata 70.016 1713 Riband Wave Idaea aversata 70.053 1722 Flame Carpet Xanthorhoe designata 70.051 1724 Red Twin-spot Carpet Xanthorhoe spadicearia 70.049 1728 Garden Carpet Xanthorhoe fluctuata 70.061 1738 Common Carpet Epirrhoe alternata 70.059 1742 Yellow Shell Camptogramma bilineata 70.087 1752 Purple Bar Cosmorhoe ocellata 70.093 1758 Barred Straw Eulithis (Gandaritis) pyraliata 70.097 1764 Common Marbled Carpet Chloroclysta truncata 70.085 1765 Barred Yellow Cidaria fulvata 70.100 1776 Green Carpet Colostygia pectinataria 70.126 1781 Small Waved Umber Horisme vitalbata 70.107 1795 November/Autumnal Moth agg Epirrita dilutata agg. -

Results of a Lepidopterological Expedition to North and Northwest Iran in Summer 2007 with New Records for Iran (Lepidoptera) (Plates 19-22)
Esperiana Band 16: 135-165 Schwanfeld, 06. Dezember 2011 ISBN 978-3-938249-01-7 Results of a lepidopterological expedition to North and Northwest Iran in summer 2007 with new records for Iran (Lepidoptera) (plates 19-22) Lutz LEHMANN † & Reza ZAHIRI Abstract: The results of a joint lepidopterological expedition to North and Northwest Iran, povinces of Tehran, Mazandaran, Guilan, Ardabil and Azerbaijan-e-Sharqi, from 20 July to 1 August are presented. More than 508 species of Macrolepidoptera (sensu SEITZ) could be recorded, among them Idaea sericeata (HÜBNER, 1813), Cinglis humifusaria EVERSMANN, 1837, Aplocera uniformata (URBAHN, 1971), Scotopteryx chenopodiata (LINNAEUS, 1758), Triphosa dubitata (LINANEUS, 1758), Abraxas grossulariata (LINNAEUS, 1758), Kemtrognophos ciscaucasica (RJABOV, 1964), Furcula danieli SCHINTLMEISTER, 1998, Eilema lurideola ([ZINCKEN], 1817), Zethes propinquus CHRISTOPH, 1885, Meganola togatulalis (HÜBNER, 1796), Meganola kolbi (DANIEL, 1935), Pseudluperina pozzii (CURÓ, 1883), Mythimna sicula scirpi (DUPONCHEL, 1836) and Noctua interposita (HÜBNER, 1790) new for the fauna of Iran. Additionally, the species of two light traps in the Elburs Mts., province Mazandaran, from the beginning of June, collected by A. PÜTZ, are listed. The genitalia of 16 species, typical habitats, and some living and spread specimens are figured. Zusammenfassung: Die Ergebnisse einer gemeinsamen lepidopterologischen Expedition in den Nord- und Nordwestiran, Provinzen Teheran, Mazandaran, Guilan, Ardabil and Azerbaijan-e-Sharqi, vom -

Evidence for Breeding Lime Hawkmoth (Mimas Tiliae) in Glasgow, Scotland
The Glasgow Naturalist (online 2020) Volume 27, Part 2 https://doi.org/10.37208/tgn27217 surprise, therefore, when a mating pair of lime hawkmoths was photographed in Glebe Street, Renfrew, Evidence for breeding lime Renfrewshire on 27th May 2016. Whilst publicised at the time on the Paisley Natural History Society website hawkmoth (Mimas tiliae) in Glasgow, (PNHS, 2020) and in the local press (Paisley Daily Scotland Express, 20th June 2016), there were no subsequent sightings. Indeed, the possibility was raised that the pair A.P. Payne could have been purchased via one of several commercial operators dealing in hawkmoths. Thomson Building, University of Glasgow, Glasgow G12 8QQ On the late afternoon of 12th August 2019 I found a large green hawkmoth caterpillar on top of a low wall in E-mail: [email protected] Orleans Avenue, Jordanhill, Glasgow (Fig. 1). Close examination revealed a blue horn on the tail with a pink blush on the undersurface of the base. The tail also bore prominent yellow warts surrounding a dark red patch. The lime hawkmoth (Mimas tiliae) is an attractive olive The spiracles were ringed in red and there were seven and sand-coloured, medium-sized hawkmoth with a oblique yellow stripes on the light green body. This was wide distribution in Europe, the Middle East and Asia subsequently confirmed as a final instar lime hawkmoth (Pittaway, 1993). There are two 19th century records of larva. the moth in the Glasgow area: one by John Dunsmore near Houston, Renfrewshire; the other by Robert The caterpillar was found immediately underneath a Dunlop near Kilmarnock, East Ayrshire. -

Nuclear Genes Carbamoyl Phosphate Synthetase And
November 8–9, 2017, Brno, Czech Republic 24 years NUCLEAR GENES CARBAMOYL PHOSPHATE SYNTHETASE AND ELONGATION FACTOR-1α AS TOOL FOR IDENTIFICATION OF INTRASPECIFIC GENE VARIATION IN CASE OF LIME HAWK-MOTH (MIMAS TILIAE) TAMARA MIFKOVA, ALES KNOLL, JAN WIJACKI Department of Morphology, Physiology and Animal Genetics Mendel University in Brno Zemedelska 1, 613 00 Brno CZECH REPUBLIC [email protected] Abstract: Within the distributional areal of Lime Haw-Moth (Mimas tiliae) probably exist several subspecies, some of them are already described (M. t. kitchingi). The aim of this pilot study was to verify usability of the nuclear genes CAD and EF-1α by the DNA barcoding method to distinguish between morphologically different subpopulations and that the DNA sequence of this genes corresponds to its geographic distribution. We have demonstrated that these CAD and EF-1α gene fragments are suitable for species identification, but not for subspecies differentiation. Relation to geographical distribution was not confirmed for CAD gene, but in case of EF-1α gene such a relationship can be seen. Key Words: sphingidae, DNA barcoding, biodiversity, hawk-moth, nuclear genes INTRODUCTION Biodiversity is an essential element to protect life on the Earth. We know three main categories of biodiversity: gene diversity, species diversity and the complex ecosystem diversity. There are estimated 10 million plant and animal species, but only about 1.5 million are described (and more than 1 million are insects) in the World. DNA barcoding is taxonomical method using a very short DNA sequences from standard part of the genome and alignmenting them with sequencing databases. Can be easily be used even for species description (e.g. -

The Spanish Pyrenees
The Spanish Pyrenees Naturetrek Tour Report 13 - 20 May 2012 Giant Peacock courtesy of Tim Crafer Group photo by Janet Blizard Berdun by Janet Blizard Report compiled by Philip Thompson Naturetrek Cheriton Mill Cheriton Alresford Hampshire SO24 0NG England T: +44 (0)1962 733051 F: +44 (0)1962 736426 E: [email protected] W: www.naturetrek.co.uk Tour REport The Spanish Pyrenees Tour Leaders:- Philip Thompson Byron Palacios Participants:- Tim Crafer Shelagh Crafer Liz Savory Alan Dawson Chris Dawson Annie Green Steve Tilbury Kate Tilbury Tim Lait Rita Lait Janet Blizard Louise Thompson Day 1 Sunday 13th May Once the group had assembled we were quickly away in our tour minibuses in the early evening heading north across the Zaragoza plains towards the Pyrenees. Several Black Kites were seen as we passed, followed by a flock of Cattle Egrets and single Marsh Harrier at a small wetland beside the motorway. We enjoyed a lovely sunset as we climbed through the pre-Pyrenean hills and then dropped down into La Canal de Berdun and got our first view of Berdun itself perched on its isolated hilltop. We enjoyed a late meal and refreshments before retiring to bed. Day 2 Monday 14th May After our long day of travel the previous day we enjoyed a pleasant walk into the ‘Badlands’ of eroded slopes below the village of Berdun down towards the Rio Veral. As we set off from the village several wayside plants were picked out which included both Erodium malacoides and ciconium and Papaver hybridum. A very obliging Nightingale sat out in the open for some time allowing everyone to get a great view of these usually very secretive and skulking birds which nonetheless are generally very common and heard singing throughout the trip. -

The Potential Impacts of Climate Change on the Biodiversity of Norfolk Jeff Price
The potential impacts of climate change on the biodiversity of Norfolk Jeff Price Introduction on a trajectory for ~3.2°C increase (UNEP Climate change is posing, and will continue 2016). While this is an improvement over to pose, increasing risks to biodiversity the previous ‘business as usual’ estimate (O’Neill et al. 2017). Changes in phenology of 4°- 4.5°C, it is still likely to have a large and range were first noted more than a impact on biodiversity. decade ago (Root et al. 2003) with many This paper reviews the projected climate publications since. Land use change is change impacts (relative to 1961-1990 increasingly a problem as species are being baseline) on some of the biodiversity further challenged by barriers to their in Norfolk (including birds, mammals, potential dispersal with their preferred reptiles, amphibians, butterflies, common climate across fragmented landscapes macro moths, dragonflies, bumblebees, (Settele et al. 2014). Many studies have grasshoppers, shieldbugs, ferns, orchids, examined the potential future impacts and some trees and shrubs. The paper of climate change on biodiversity using concentrates on the species currently found a variety of modelling techniques. This in Norfolk (largely based on lists on the includes results from Wallace Initiative Norfolk and Norwich Naturalist’s Society Phase 1 models showing the potential for website) and not on potential colonists range losses of greater than 50% across large from Europe. The exception is for some fractions of species globally at warming of the birds and dragonflies. For brevity levels of approximately 3.6 °C above pre- it concentrates on the climate changes industrial levels (Warren et al. -

BIODIVERSITY and ENVIRONMENT of NEW ROAD, LITTLE LONDON and NEIGHBOURING COUNTRYSIDE by Dr Paul Sterry Contents: 1
BIODIVERSITY AND ENVIRONMENT OF NEW ROAD, LITTLE LONDON AND NEIGHBOURING COUNTRYSIDE by Dr Paul Sterry Contents: 1. Summary. 2. A brief history. 3. Notable habitats alongside New Road and in the neighbouring countryside. 4. Protected and notable species found on New Road and in the surrounding countryside. Appendix 1 - Historical land use in Little London and its influence on biodiversity. Appendix 2 - Lepidoptera (Butterflies and Moths) recorded on New Road, Little London 2004-2019 (generalised OS Grid Reference SU6159). Appendix 3 - Ageing Hedgerows. About the author : Paul Sterry has BSc and PhD in Zoology and Ecology from Imperial College, London. After 5 years as a Research Fellow at the University of Sussex working on freshwater ecology he embarked on a freelance career as a wildlife author and photographer. Over the last 35 years he has written and illustrated more than 50 books, concentrating mainly on British Wildlife, with the emphasis on photographic field guides. Best-selling titles include Collins Complete British Trees, Collins Complete British Wildlife and Collins Life-size Birds. Above: Barn Owl flying over grassland in the neighbourhood of New Road. 1. Summary Located in the Parish of Pamber, Little London is a Biodiversity hotspot with New Road at its environmental heart. Despite the name New Road is one of the oldest highways in the village and this is reflected in the range of wildlife found along its length, and in the countryside bordering it. New Road has significance for wildlife far beyond is narrow, single-track status. Its ancient hedgerows and adjacent meadows are rich in wildlife but of equal importance is its role as a corridor of wildlife connectivity. -

Introductory Chapter: Moths
Chapter 1 Introductory Chapter: Moths Farzana Khan PerveenKhan Perveen and Anzela KhanAnzela Khan Additional information is available at the end of the chapter http://dx.doi.org/10.5772/intechopen.79639 1. Moths The moths (Insecta: Lepidoptera) are the group of organisms allied to butterflies, having two pairs of wide wings shielded with microscopic scales. They are usually brightly colored and held flat at sitting posture. The word moths are derived from Scandinavian word mott, for maggot, perhaps a reference to the caterpillars of moths. Furthermore, about 165,000 species of moths, including micro- and macro-moths are found worldwide, many of which are yet to be described (Table 1) [1–3]. Kingdom: Animalia Subkingdom: Invertebrata Super-Division: Eumetazoa Division: Bilateria Subdivision: Ecdysozoa Superphylum: Tactopoda Phylum: Arthropoda Von Siebold, 1848 Subphylum: Atelocerata Superclass: Hexapoda Class: Insecta Infraclass: Neoptera Subclass: Pterygota Superorder: Endopterygota Unranked: Amphiesmenoptera © 2016 The Author(s). Licensee InTech. This chapter is distributed under the terms of the Creative Commons © 2018 The Author(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. distribution, and reproduction in any medium, provided the original work is properly cited. 4 Moths - Pests of Potato, Maize and Sugar Beet Unranked: Holometabola Order: Lepidoptera Linnaeus, 1758 Examples: • Micro-moths • Macro-moths Table 1. Taxonomic rank of moths [1]. 1.1. History Moths evolved long before butterflies, fossils have been found in Germany may be 200 million years old in the early Jurassic Period. -
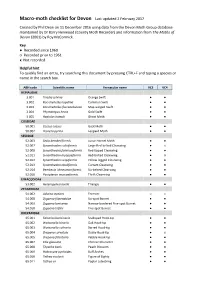
Macro-Moth Checklist for Devon Last Updated 2 February 2017
Macro-moth checklist for Devon Last updated 2 February 2017 Created by Phil Dean on 11 December 2016 using data from the Devon Moth Group database maintained by Dr Barry Henwood (County Moth Recorder) and information from The Moths of Devon (2001) by Roy McCormick. Key ● Recorded since 1960 ○ Recorded prior to 1961 x Not recorded Helpful hint To quickly find an entry, try searching this document by pressing CTRL+F and typing a species or name in the search box. ABH code Scientific name Vernacular name VC3 VC4 HEPIALIDAE 3.001 Triodia sylvina Orange Swift ● ● 3.002 Korscheltellus lupulina Common Swift ● ● 3.003 Korscheltellus fusconebulosa Map-winged Swift ● ● 3.004 Phymatopus hecta Gold Swift ● ● 3.005 Hepialus humuli Ghost Moth ● ● COSSIDAE 50.001 Cossus cossus Goat Moth ● ● 50.002 Zeuzera pyrina Leopard Moth ● ● SESIIDAE 52.003 Sesia bembeciformis Lunar Hornet Moth ● ● 52.007 Synanthedon culiciformis Large Red-belted Clearwing ● x 52.008 Synanthedon formicaeformis Red-tipped Clearwing ● ● 52.011 Synanthedon myopaeformis Red-belted Clearwing ● ○ 52.012 Synanthedon vespiformis Yellow-legged Clearwing ● ● 52.013 Synanthedon tipuliformis Currant Clearwing ● ● 52.014 Bembecia ichneumoniformis Six-belted Clearwing ● ● 52.016 Pyropteron muscaeformis Thrift Clearwing ● ● LIMACODIDAE 53.002 Heterogenea asella Triangle ● ● ZYGAENIDAE 54.002 Adscita statices Forester ○ x 54.008 Zygaena filipendulae Six-spot Burnet ● ● 54.009 Zygaena lonicerae Narrow-bordered Five-spot Burnet ● ● 54.010 Zygaena trifolii Five-spot Burnet ● ● DREPANIDAE 65.001 Falcaria -
Download (6MB)
Evolution of Variation in Plumage and Ornamentation in Least Auklets Aethia pusilla (Pallas) Martin Renner A thesis submitted in partial fulfilment for the degree of Doctor of Philosophy Memorial University of Newfoundland Canada February 2005 Library and Bibliotheque et 1+1 Archives Canada Archives Canada Published Heritage Direction du Branch Patrimoine de !'edition 395 Wellington Street 395, rue Wellington Ottawa ON K1A ON4 Ottawa ON K1A ON4 Canada Canada Your file Votre reference ISBN: 978-0-494-15661-2 Our file Notre reference ISBN: 978-0-494-15661-2 NOTICE: AVIS: The author has granted a non L'auteur a accorde une licence non exclusive exclusive license allowing Library permettant a Ia Bibliotheque et Archives and Archives Canada to reproduce, Canada de reproduire, publier, archiver, publish, archive, preserve, conserve, sauvegarder, conserver, transmettre au public communicate to the public by par telecommunication ou par !'Internet, preter, telecommunication or on the Internet, distribuer et vendre des theses partout dans loan, distribute and sell theses le monde, a des fins commerciales ou autres, worldwide, for commercial or non sur support microforme, papier, electronique commercial purposes, in microform, et/ou autres formats. paper, electronic and/or any other formats. The author retains copyright L'auteur conserve Ia propriete du droit d'auteur ownership and moral rights in et des droits moraux qui protege cette these. this thesis. Neither the thesis Ni Ia these ni des extraits substantiels de nor substantial extracts from it celle-ci ne doivent etre imprimes ou autrement may be printed or otherwise reproduits sans son autorisation. reproduced without the author's permission.