Phylogenetic Analysis of Phenotypic Characters of Tunicata Supports Basal Appendicularia and Monophyletic Ascidiacea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chordata, Tunicata, Thaliacea, Doliolida) from East Coast of Peninsular Malaysia), with an Updated Worldwide Distribution
Journal of Sustainability Science and Management ISSN: 1823-8556 Volume 13 Number 5, 2018 © Penerbit UMT TAXONOMIC REVISION OF THE FAMILY DOLIOLIDAE BRONN, 1862 (CHORDATA, TUNICATA, THALIACEA, DOLIOLIDA) FROM EAST COAST OF PENINSULAR MALAYSIA), WITH AN UPDATED WORLDWIDE DISTRIBUTION NUR ‘ALIAH BINTI ADAM1 AND NURUL HUDA AHMAD ISHAK*1, 2 1School of Marine and Environmental Sciences, Universiti Malaysia Terengganu, 21030 Kuala Nerus, Terengganu, Malaysia 2Institute of Oceanography and Environment, Universiti Malaysia Terengganu, 21030 Kuala Nerus, Terengganu, Malaysia *Corresponding author: [email protected] Abstract: The marine pelagic tunicate from the family of Doliolidae Bronn, 1862 in the coastal waters of Terengganu was studied for the first time, hereby presented in this paper. The distribution was analysed from 18 sampling stations alongside the Terengganu waters; including Pulau Bidong, Pulau Yu and Pulau Kapas. Samples were collected from April to July 2016 using 200µm Bongo net; towed vertically from a stationary vessel; and were preserved in a 5% buffered formaldehyde. Five species discovered in this family were identified as new records in Malaysian waters:Doliolum denticulatum Quoy and Gaimard, 1834, Doliolum nationalis Borgert, 1894, Dolioletta gegenbauri Uljanin, 1884, Doliolina mulleri Krohn, 1852 and Dolioloides rarum Grobben, 1882. A comprehensive review of the species description, diagnosis and a key to the phorozooid from the recorded species is herewith provided. We also deliver a detailed map of current and known worldwide occurrence of these five species, and thus consequently update the biodiversity of Malaysian fauna. KEYWORDS: Doliolid, pelagic tunicates, South China Sea, Terengganu, taxonomy, biogeography Introduction have the most complex life cycle compared to any of the pelagic tunicates; consisting of no lesser Pelagic tunicates are large transparent animals than six different and successive morphological that measure up to 25cm (Lavaniegos & Ohman, stages (Godeaux et al., 1998; Paffenhöfer & 2003). -
Appendicularia of CTAW
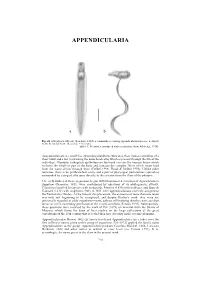
APPENDICULARIA APPENDICULARIA a b Fig. 26. Oikopleura albicans (Leuckart, 1854), a commonly occurring appendicularian species: a, dorsal view; b, lateral view. (Scale bar = 0.1 mm). [after T. Prentiss, reproduced with permission, from Alldredge 1976] Appendicularians are small free swimming planktonic tunicates, their bodies consisting of a short trunk and a tail (containing the notochord cells) which is present through the life of the individual. Glandular (oikoplast) epithelium on the trunk secretes the mucous house which encloses the whole or part of the body and contains the complex filters which strain food from the water driven through them (Deibel 1998; Flood & Deibel 1998). Unlike other tunicates, there is no peribranchial cavity and a pair of pharyngeal perforations (spiracles) surrounded by a ring of cilia open directly to the exterior from the floor of the pharynx. The early studies of these organisms, begun with Chamisso's description of Appendicularia flagellum Chamisso, 1821, were confounded by questions of its phylogenetic affinity. Chamisso classified his species with medusoids, Mertens (1830) with molluscs, and Quoy & Gaimard (1833) with zoophytes. Only in 1851 were appendicularians correctly assigned to the Tunicata by Huxley. At the time of this placement, the existence of more than one taxon was only just beginning to be recognised, and despite Huxley's work, they were not universally regarded as adult organisms—some authors still insisting that they were ascidian larvae or a free swimming generation of the sessile ascidians (Fenaux 1993). Subsequently, these questions were resolved by the work of Fol (1872) on material from the Straits of Messina, which forms the basis of later studies on the large collections of the great expeditions of the 19th century that revealed their true diversity in the oceanic plankton. -

Brains of Primitive Chordates 439
Brains of Primitive Chordates 439 Brains of Primitive Chordates J C Glover, University of Oslo, Oslo, Norway although providing a more direct link to the evolu- B Fritzsch, Creighton University, Omaha, NE, USA tionary clock, is nevertheless hampered by differing ã 2009 Elsevier Ltd. All rights reserved. rates of evolution, both among species and among genes, and a still largely deficient fossil record. Until recently, it was widely accepted, both on morpholog- ical and molecular grounds, that cephalochordates Introduction and craniates were sister taxons, with urochordates Craniates (which include the sister taxa vertebrata being more distant craniate relatives and with hemi- and hyperotreti, or hagfishes) represent the most chordates being more closely related to echinoderms complex organisms in the chordate phylum, particu- (Figure 1(a)). The molecular data only weakly sup- larly with respect to the organization and function of ported a coherent chordate taxon, however, indicat- the central nervous system. How brain complexity ing that apparent morphological similarities among has arisen during evolution is one of the most chordates are imposed on deep divisions among the fascinating questions facing modern science, and it extant deuterostome taxa. Recent analysis of a sub- speaks directly to the more philosophical question stantially larger number of genes has reversed the of what makes us human. Considerable interest has positions of cephalochordates and urochordates, pro- therefore been directed toward understanding the moting the latter to the most closely related craniate genetic and developmental underpinnings of nervous relatives (Figure 1(b)). system organization in our more ‘primitive’ chordate relatives, in the search for the origins of the vertebrate Comparative Appearance of Brains, brain in a common chordate ancestor. -

Eudistoma (Ascidiacea: Polycitoridae) from Tropical Brazil
ZOOLOGIA 31 (2): 195–208, April, 2014 http://dx.doi.org/10.1590/S1984-46702014000200011 Eudistoma (Ascidiacea: Polycitoridae) from tropical Brazil Livia de Moura Oliveira1, Gustavo Antunes Gamba1 & Rosana Moreira da Rocha1,2 1 Programa de Pós-graduação em Zoologia, Departamento de Zoologia, Universidade Federal do Paraná. Caixa Postal 19020, 81531-980 Curitiba, PR, Brazil. 2 Corresponding author: E-mail: [email protected] ABSTRACT. We studied material in collections from coastal intertidal and subtidal tropical waters of the Brazilian states of Paraíba, Pernambuco, Alagoas, Bahia, and Espírito Santo. We identified seven species of Eudistoma, of which two are new to science. Eudistoma alvearium sp. nov. colonies have fecal pellets around each zooid and zooids are 6-8 mm long with seven straight and parallel pyloric tubules; the larval trunk is 0.6 mm long with three adhesive papillae and ten ampullae. Eudistoma versicolor sp. nov. colonies are cushion-shaped, variable in color (blue, purple, brown, light green, gray or white) and zooids have six straight and parallel pyloric tubules; the larval trunk is 0.8 mm long with three adhesive papillae and six ampules. Three species – E. carolinense Van Name, 1945, E. recifense Millar, 1977, and E. vannamei Millar, 1977 – are known from northeastern Brazil. The identification of two additional species will require confirmation. We also propose a synonymy for E. carolinense with E. repens Millar, 1977, also previously described in Brazil. KEY WORDS. Atlantic; colonial ascidians; new species; taxonomy. Eudistoma Caullery, 1909 is the most species-rich genus and comment on the implications of species richness for the in Polycitoridae, with 124 valid species found in tropical and distribution of Eudistoma. -

The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom
lopmen ve ta e l B Williamson, Cell Dev Biol 2012, 1:1 D io & l l o l g DOI: 10.4172/2168-9296.1000101 e y C Cell & Developmental Biology ISSN: 2168-9296 Research Article Open Access The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom Abstract The larval transfer hypothesis states that larvae originated as adults in other taxa and their genomes were transferred by hybridization. It contests the view that larvae and corresponding adults evolved from common ancestors. The present paper reviews the life histories of chordates, and it interprets them in terms of the larval transfer hypothesis. It is the first paper to apply the hypothesis to craniates. I claim that the larvae of tunicates were acquired from adult larvaceans, the larvae of lampreys from adult cephalochordates, the larvae of lungfishes from adult craniate tadpoles, and the larvae of ray-finned fishes from other ray-finned fishes in different families. The occurrence of larvae in some fishes and their absence in others is correlated with reproductive behavior. Adult amphibians evolved from adult fishes, but larval amphibians did not evolve from either adult or larval fishes. I submit that [1] early amphibians had no larvae and that several families of urodeles and one subfamily of anurans have retained direct development, [2] the tadpole larvae of anurans and urodeles were acquired separately from different Mesozoic adult tadpoles, and [3] the post-tadpole larvae of salamanders were acquired from adults of other urodeles. Reptiles, birds and mammals probably evolved from amphibians that never acquired larvae. -

The Plankton Lifeform Extraction Tool: a Digital Tool to Increase The
Discussions https://doi.org/10.5194/essd-2021-171 Earth System Preprint. Discussion started: 21 July 2021 Science c Author(s) 2021. CC BY 4.0 License. Open Access Open Data The Plankton Lifeform Extraction Tool: A digital tool to increase the discoverability and usability of plankton time-series data Clare Ostle1*, Kevin Paxman1, Carolyn A. Graves2, Mathew Arnold1, Felipe Artigas3, Angus Atkinson4, Anaïs Aubert5, Malcolm Baptie6, Beth Bear7, Jacob Bedford8, Michael Best9, Eileen 5 Bresnan10, Rachel Brittain1, Derek Broughton1, Alexandre Budria5,11, Kathryn Cook12, Michelle Devlin7, George Graham1, Nick Halliday1, Pierre Hélaouët1, Marie Johansen13, David G. Johns1, Dan Lear1, Margarita Machairopoulou10, April McKinney14, Adam Mellor14, Alex Milligan7, Sophie Pitois7, Isabelle Rombouts5, Cordula Scherer15, Paul Tett16, Claire Widdicombe4, and Abigail McQuatters-Gollop8 1 10 The Marine Biological Association (MBA), The Laboratory, Citadel Hill, Plymouth, PL1 2PB, UK. 2 Centre for Environment Fisheries and Aquacu∑lture Science (Cefas), Weymouth, UK. 3 Université du Littoral Côte d’Opale, Université de Lille, CNRS UMR 8187 LOG, Laboratoire d’Océanologie et de Géosciences, Wimereux, France. 4 Plymouth Marine Laboratory, Prospect Place, Plymouth, PL1 3DH, UK. 5 15 Muséum National d’Histoire Naturelle (MNHN), CRESCO, 38 UMS Patrinat, Dinard, France. 6 Scottish Environment Protection Agency, Angus Smith Building, Maxim 6, Parklands Avenue, Eurocentral, Holytown, North Lanarkshire ML1 4WQ, UK. 7 Centre for Environment Fisheries and Aquaculture Science (Cefas), Lowestoft, UK. 8 Marine Conservation Research Group, University of Plymouth, Drake Circus, Plymouth, PL4 8AA, UK. 9 20 The Environment Agency, Kingfisher House, Goldhay Way, Peterborough, PE4 6HL, UK. 10 Marine Scotland Science, Marine Laboratory, 375 Victoria Road, Aberdeen, AB11 9DB, UK. -

Aliens in Egyptian Waters. a Checklist of Ascidians of the Suez Canal and the Adjacent Mediterranean Waters
Egyptian Journal of Aquatic Research (2016) xxx, xxx–xxx HOSTED BY National Institute of Oceanography and Fisheries Egyptian Journal of Aquatic Research http://ees.elsevier.com/ejar www.sciencedirect.com FULL LENGTH ARTICLE Aliens in Egyptian waters. A checklist of ascidians of the Suez Canal and the adjacent Mediterranean waters Y. Halim a, M. Abdel Messeih b,* a Oceanography Department, Faculty of Science, Alexandria, Egypt b National Institute of Oceanography and Fisheries, Alexandria, Egypt Received 3 April 2016; revised 21 August 2016; accepted 22 August 2016 KEYWORDS Abstract Checklists of the alien ascidian fauna of Egyptian waters are provided covering the Suez Ascidians; Canal, the adjacent Mediterranean waters and the Gulf of Suez. Enrichment in ascidian species of Mediterranean Sea; the Suez Canal seems to have been on the increase since 1927. The distinctly uneven distribution Erythrean non-indigenous pattern in the Canal appears to be directly related to the ship traffic system. species; Earlier reports on alien ascidian species in the Mediterranean are compared and discussed. Of 65 Suez Canal; species recorded from the Mediterranean waters of Egypt in all, four are Erythrean migrants and Polyclinum constellatum four potentially so. Polyclinum constellatum Savigny, 1816 is a new record for the Mediterranean Sea. Ó 2016 National Institute of Oceanography and Fisheries. Hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). Introduction 2005 and 2014 to deal with this issue and with other related problems. Ascidians are receiving more and more attention because of Based on an analysis of the literature and on the on-line the invasive ability of some species and the severe damage World Register of Marine Species (www.marinespecies.org/), caused to aquaculture (reviewed in a special issue of Aquatic Shenkar and Swalla (2011) assembled 2815 described ascidian Invasions, January 2009: http://aquatic invasions.net/2009/in- species. -

1471-2148-9-187.Pdf
BMC Evolutionary Biology BioMed Central Research article Open Access An updated 18S rRNA phylogeny of tunicates based on mixture and secondary structure models Georgia Tsagkogeorga1,2, Xavier Turon3, Russell R Hopcroft4, Marie- Ka Tilak1,2, Tamar Feldstein5, Noa Shenkar5,6, Yossi Loya5, Dorothée Huchon5, Emmanuel JP Douzery1,2 and Frédéric Delsuc*1,2 Address: 1Université Montpellier 2, Institut des Sciences de l'Evolution (UMR 5554), CC064, Place Eugène Bataillon, 34095 Montpellier Cedex 05, France, 2CNRS, Institut des Sciences de l'Evolution (UMR 5554), CC064, Place Eugène Bataillon, 34095 Montpellier Cedex 05, France, 3Centre d'Estudis Avançats de Blanes (CEAB, CSIC), Accés Cala S. Francesc 14, 17300 Blanes (Girona), Spain, 4Institute of Marine Science, University of Alaska Fairbanks, Fairbanks, Alaska, USA, 5Department of Zoology, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv, 69978, Israel and 6Department of Biology, University of Washington, Seattle WA 98195, USA Email: Georgia Tsagkogeorga - [email protected]; Xavier Turon - [email protected]; Russell R Hopcroft - [email protected]; Marie-Ka Tilak - [email protected]; Tamar Feldstein - [email protected]; Noa Shenkar - [email protected]; Yossi Loya - [email protected]; Dorothée Huchon - [email protected]; Emmanuel JP Douzery - [email protected]; Frédéric Delsuc* - [email protected] * Corresponding author Published: 5 August 2009 Received: 16 October 2008 Accepted: 5 August 2009 BMC Evolutionary Biology 2009, 9:187 doi:10.1186/1471-2148-9-187 This article is available from: http://www.biomedcentral.com/1471-2148/9/187 © 2009 Tsagkogeorga et al; licensee BioMed Central Ltd. -

DEEP SEA LEBANON RESULTS of the 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project
DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project March 2018 DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project Citation: Aguilar, R., García, S., Perry, A.L., Alvarez, H., Blanco, J., Bitar, G. 2018. 2016 Deep-sea Lebanon Expedition: Exploring Submarine Canyons. Oceana, Madrid. 94 p. DOI: 10.31230/osf.io/34cb9 Based on an official request from Lebanon’s Ministry of Environment back in 2013, Oceana has planned and carried out an expedition to survey Lebanese deep-sea canyons and escarpments. Cover: Cerianthus membranaceus © OCEANA All photos are © OCEANA Index 06 Introduction 11 Methods 16 Results 44 Areas 12 Rov surveys 16 Habitat types 44 Tarablus/Batroun 14 Infaunal surveys 16 Coralligenous habitat 44 Jounieh 14 Oceanographic and rhodolith/maërl 45 St. George beds measurements 46 Beirut 19 Sandy bottoms 15 Data analyses 46 Sayniq 15 Collaborations 20 Sandy-muddy bottoms 20 Rocky bottoms 22 Canyon heads 22 Bathyal muds 24 Species 27 Fishes 29 Crustaceans 30 Echinoderms 31 Cnidarians 36 Sponges 38 Molluscs 40 Bryozoans 40 Brachiopods 42 Tunicates 42 Annelids 42 Foraminifera 42 Algae | Deep sea Lebanon OCEANA 47 Human 50 Discussion and 68 Annex 1 85 Annex 2 impacts conclusions 68 Table A1. List of 85 Methodology for 47 Marine litter 51 Main expedition species identified assesing relative 49 Fisheries findings 84 Table A2. List conservation interest of 49 Other observations 52 Key community of threatened types and their species identified survey areas ecological importanc 84 Figure A1. -

Ascidians from the Tropical Western Pacific
Ascidians from the tropical western Pacific Françoise MONNIOT Claude MONNIOT CNRS UPESA 8044, Laboratoire de Biologie des Invertébrés marins et Malacologie, Muséum national d’Histoire naturelle, 55 rue Buffon, F-75005 Paris (France) [email protected] Monniot F. & Monniot C. 2001. — Ascidians from the tropical western Pacific. Zoosystema 23 (2) : 201-383. ABSTRACT A large collection of 187 identified ascidian species is added to the records published in 1996 from the same tropical western Pacific islands. Most of the specimens were collected by the US Coral Reef Research Foundation (CRRF). They come from depths accessible by SCUBA diving. Most of the collection’s species are described and figured, their color in life is illustrated by 112 underwater photographs; among them 48 are new species represent- ing a fourth of the material collected. This demonstrates how incomplete the knowledge of the ascidian diversity in this part of the world remains. Moreover, very small or inconspicuous species were seldom collected, as com- pared to the highly coloured and large forms. Many other immature speci- mens were also collected but their precise identification was not possible. Almost all littoral families are represented with the exception of the Molgulidae which are more characteristic of soft sediments, a biotope which was not investigated. Among the very diversified genera, the colonial forms largely dominate, including not only all the Aplousobranchia genera but also some Phlebobranchia and Stolidobranchia. Very often only one specimen was KEY WORDS Tunicata, available, so a detailed biogeographical distribution cannot be given, and no western Pacific Ocean. island endemism can be defined. ZOOSYSTEMA • 2001 • 23 (2) © Publications Scientifiques du Muséum national d’Histoire naturelle, Paris. -

Marine Biology
Marine Biology Spatial and temporal dynamics of ascidian invasions in the continental United States and Alaska. --Manuscript Draft-- Manuscript Number: MABI-D-16-00297 Full Title: Spatial and temporal dynamics of ascidian invasions in the continental United States and Alaska. Article Type: S.I. : Invasive Species Keywords: ascidians, biofouling, biogeography, marine invasions, nonindigenous, non-native species, North America Corresponding Author: Christina Simkanin, Phd Smithsonian Environmental Research Center Edgewater, MD UNITED STATES Corresponding Author Secondary Information: Corresponding Author's Institution: Smithsonian Environmental Research Center Corresponding Author's Secondary Institution: First Author: Christina Simkanin, Phd First Author Secondary Information: Order of Authors: Christina Simkanin, Phd Paul W. Fofonoff Kristen Larson Gretchen Lambert Jennifer Dijkstra Gregory M. Ruiz Order of Authors Secondary Information: Funding Information: California Department of Fish and Wildlife Dr. Gregory M. Ruiz National Sea Grant Program Dr. Gregory M. Ruiz Prince William Sound Regional Citizens' Dr. Gregory M. Ruiz Advisory Council Smithsonian Institution Dr. Gregory M. Ruiz United States Coast Guard Dr. Gregory M. Ruiz United States Department of Defense Dr. Gregory M. Ruiz Legacy Program Abstract: SSpecies introductions have increased dramatically in number, rate, and magnitude of impact in recent decades. In marine systems, invertebrates are the largest and most diverse component of coastal invasions throughout the world. Ascidians are conspicuous and well-studied members of this group, however, much of what is known about their invasion history is limited to particular species or locations. Here, we provide a large-scale assessment of invasions, using an extensive literature review and standardized field surveys, to characterize the invasion dynamics of non-native ascidians in the continental United States and Alaska. -

Ascidiacea (Chordata: Tunicata) of Greece: an Updated Checklist
Biodiversity Data Journal 4: e9273 doi: 10.3897/BDJ.4.e9273 Taxonomic Paper Ascidiacea (Chordata: Tunicata) of Greece: an updated checklist Chryssanthi Antoniadou‡, Vasilis Gerovasileiou§§, Nicolas Bailly ‡ Department of Zoology, School of Biology, Aristotle University of Thessaloniki, Thessaloniki, Greece § Institute of Marine Biology, Biotechnology and Aquaculture, Hellenic Centre for Marine Research, Heraklion, Greece Corresponding author: Chryssanthi Antoniadou ([email protected]) Academic editor: Christos Arvanitidis Received: 18 May 2016 | Accepted: 17 Jul 2016 | Published: 01 Nov 2016 Citation: Antoniadou C, Gerovasileiou V, Bailly N (2016) Ascidiacea (Chordata: Tunicata) of Greece: an updated checklist. Biodiversity Data Journal 4: e9273. https://doi.org/10.3897/BDJ.4.e9273 Abstract Background The checklist of the ascidian fauna (Tunicata: Ascidiacea) of Greece was compiled within the framework of the Greek Taxon Information System (GTIS), an application of the LifeWatchGreece Research Infrastructure (ESFRI) aiming to produce a complete checklist of species recorded from Greece. This checklist was constructed by updating an existing one with the inclusion of recently published records. All the reported species from Greek waters were taxonomically revised and cross-checked with the Ascidiacea World Database. New information The updated checklist of the class Ascidiacea of Greece comprises 75 species, classified in 33 genera, 12 families, and 3 orders. In total, 8 species have been added to the previous species list (4 Aplousobranchia, 2 Phlebobranchia, and 2 Stolidobranchia). Aplousobranchia was the most speciose order, followed by Stolidobranchia. Most species belonged to the families Didemnidae, Polyclinidae, Pyuridae, Ascidiidae, and Styelidae; these 4 families comprise 76% of the Greek ascidian species richness. The present effort revealed the limited taxonomic research effort devoted to the ascidian fauna of Greece, © Antoniadou C et al.