Brains of Primitive Chordates 439
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Phylum Vertebrata: a Case for Zoological Recognition Naoki Irie1,2* , Noriyuki Satoh3 and Shigeru Kuratani4
Irie et al. Zoological Letters (2018) 4:32 https://doi.org/10.1186/s40851-018-0114-y REVIEW Open Access The phylum Vertebrata: a case for zoological recognition Naoki Irie1,2* , Noriyuki Satoh3 and Shigeru Kuratani4 Abstract The group Vertebrata is currently placed as a subphylum in the phylum Chordata, together with two other subphyla, Cephalochordata (lancelets) and Urochordata (ascidians). The past three decades, have seen extraordinary advances in zoological taxonomy and the time is now ripe for reassessing whether the subphylum position is truly appropriate for vertebrates, particularly in light of recent advances in molecular phylogeny, comparative genomics, and evolutionary developmental biology. Four lines of current research are discussed here. First, molecular phylogeny has demonstrated that Deuterostomia comprises Ambulacraria (Echinodermata and Hemichordata) and Chordata (Cephalochordata, Urochordata, and Vertebrata), each clade being recognized as a mutually comparable phylum. Second, comparative genomic studies show that vertebrates alone have experienced two rounds of whole-genome duplication, which makes the composition of their gene family unique. Third, comparative gene-expression profiling of vertebrate embryos favors an hourglass pattern of development, the most conserved stage of which is recognized as a phylotypic period characterized by the establishment of a body plan definitively associated with a phylum. This mid-embryonic conservation is supported robustly in vertebrates, but only weakly in chordates. Fourth, certain complex patterns of body plan formation (especially of the head, pharynx, and somites) are recognized throughout the vertebrates, but not in any other animal groups. For these reasons, we suggest that it is more appropriate to recognize vertebrates as an independent phylum, not as a subphylum of the phylum Chordata. -

Acid-Base Regulation in the Atlantic Hagfish Myxine Glutinosa
/. exp. Biol. 161, 201-215 (1991) 201 Printed in Great Britain © The Company of Biologists Limited 1991 ACID-BASE REGULATION IN THE ATLANTIC HAGFISH MYXINE GLUTINOSA BY D. G. McDONALD*, V. CAVDEK*, L. CALVERT* AND C. L. MLLLIGANf Huntsman Marine Science Centre, St Andrews, New Brunswick, Canada Accepted 10 July 1991 Summary Blood acid-base status and net transfers of acidic equivalents to the external environment were studied in hagfish, Myxine glutinosa, infused with ammonium sulphate (4mequivkg~1 NH4+) or with sulphuric acid (Smequivkg"1 H+). Hagfish extracellular fluids (ECF) play a greater role in acid-base regulation than in teleosts. This is because hagfish have a much larger blood volume relative to teleosts, despite a relatively low blood buffering capacity. Consequently, infusion of ammonium sulphate produced only half of the acidosis produced in marine teleosts in comparable studies, and hagfish readily tolerated a threefold greater direct H+ load. Furthermore, the H+ load was largely retained and buffered in the extracellular space. Despite smaller acid-base disturbances, rates of net H+ excretion to the external environment were, nonetheless, comparable to those of marine teleosts, and net acid excretion persisted until blood acid-base disturb- ances were corrected. We conclude that the gills of the hagfish are at least as competent for acid-base regulation as those of marine teleosts. The nature of the H+ excretion mechanism is discussed. Introduction + + In 1984, Evans found evidence for Na /H and C1~/HCO3~ exchanges in the primitive osmoconforming hagfish Myxine glutinosa L., and was the first to suggest that acid-base regulation by the gills preceded osmoregulation in the evolution of the vertebrates. -
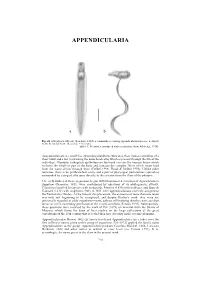
Appendicularia of CTAW
APPENDICULARIA APPENDICULARIA a b Fig. 26. Oikopleura albicans (Leuckart, 1854), a commonly occurring appendicularian species: a, dorsal view; b, lateral view. (Scale bar = 0.1 mm). [after T. Prentiss, reproduced with permission, from Alldredge 1976] Appendicularians are small free swimming planktonic tunicates, their bodies consisting of a short trunk and a tail (containing the notochord cells) which is present through the life of the individual. Glandular (oikoplast) epithelium on the trunk secretes the mucous house which encloses the whole or part of the body and contains the complex filters which strain food from the water driven through them (Deibel 1998; Flood & Deibel 1998). Unlike other tunicates, there is no peribranchial cavity and a pair of pharyngeal perforations (spiracles) surrounded by a ring of cilia open directly to the exterior from the floor of the pharynx. The early studies of these organisms, begun with Chamisso's description of Appendicularia flagellum Chamisso, 1821, were confounded by questions of its phylogenetic affinity. Chamisso classified his species with medusoids, Mertens (1830) with molluscs, and Quoy & Gaimard (1833) with zoophytes. Only in 1851 were appendicularians correctly assigned to the Tunicata by Huxley. At the time of this placement, the existence of more than one taxon was only just beginning to be recognised, and despite Huxley's work, they were not universally regarded as adult organisms—some authors still insisting that they were ascidian larvae or a free swimming generation of the sessile ascidians (Fenaux 1993). Subsequently, these questions were resolved by the work of Fol (1872) on material from the Straits of Messina, which forms the basis of later studies on the large collections of the great expeditions of the 19th century that revealed their true diversity in the oceanic plankton. -

(= Amphioxus) Branchiostoma Floridae
MARINE ECOLOGY PROGRESS SERIES Vol. 130: 71-84,1996 Published January 11 Mar Ecol Prog Ser Larval settlement, post-settlement growth and secondary production of the Florida lancelet (= amphioxus) Branchiostoma floridae M. D. Stokes* Marine Biology Research Division, Scripps Institution of Oceanography, La Jolla, California 92093-0202, USA ABSTRACT A population of Branch~ostomaflondae in Tampa Bay, Flonda, USA was sieved from the substratum frequently (often daily) from June 1992 through September 1994 Body lengths were mea- sured for 54264 luvenlle and adult lancelets The breedlng season lasted each year from early May through early September and newly metamorphosed lancelets settled as luveniles from late May through mid October, dunng this period of the year dlstinct settlements occurred approxmately every 1 to 3 wk Post-settlement growth was followed as changes in modal length on size-frequency histo- grams Changes in cohort growth over this peliod were compared to several different simple and seasonally oscillating growth models The von Bertalanffy functlon in smple and oscillating forms provided the best estmates of lancelet growth The lancelets grew in summer (almost 0 5 mm d-' in recently settled luveniles), but growth slowed and almost ceased durlng wlnter B flondae can llve at least 2 yr and can reach a maxlmum length of 58 mm The maximal secondary productlon was 61 53 g m-' yrrl (ash-free dry welght) and the productlon to biomass ratio was 11 64 Population den- sities at the study site ranged from about 100 to 1200 lancelets m ' KEY WORDS: Lancelet . Amphioxus . Branchiostorna flondae . Growth . Production . Breeding season . -

The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom
lopmen ve ta e l B Williamson, Cell Dev Biol 2012, 1:1 D io & l l o l g DOI: 10.4172/2168-9296.1000101 e y C Cell & Developmental Biology ISSN: 2168-9296 Research Article Open Access The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom Abstract The larval transfer hypothesis states that larvae originated as adults in other taxa and their genomes were transferred by hybridization. It contests the view that larvae and corresponding adults evolved from common ancestors. The present paper reviews the life histories of chordates, and it interprets them in terms of the larval transfer hypothesis. It is the first paper to apply the hypothesis to craniates. I claim that the larvae of tunicates were acquired from adult larvaceans, the larvae of lampreys from adult cephalochordates, the larvae of lungfishes from adult craniate tadpoles, and the larvae of ray-finned fishes from other ray-finned fishes in different families. The occurrence of larvae in some fishes and their absence in others is correlated with reproductive behavior. Adult amphibians evolved from adult fishes, but larval amphibians did not evolve from either adult or larval fishes. I submit that [1] early amphibians had no larvae and that several families of urodeles and one subfamily of anurans have retained direct development, [2] the tadpole larvae of anurans and urodeles were acquired separately from different Mesozoic adult tadpoles, and [3] the post-tadpole larvae of salamanders were acquired from adults of other urodeles. Reptiles, birds and mammals probably evolved from amphibians that never acquired larvae. -

Travels on the Backbone Crest (A Group of Embryonic Cells)
BOOKS & ARTS COMMENT EVOLUTION non-deuterostomes. In the most effective section, he strips vertebrates down to their parts, such as the nerve cord, notochord (fore- runner of the vertebral column) and neural Travels on the backbone crest (a group of embryonic cells). He even delves into the largely ignored gut and viscera. Chris Lowe lauds a study of vertebrate origins that Gee discusses how data from living and brings us up to date with a shifting field. fossilized hemichordates and echinoderms have facilitated the search for the origins of the defining chordate anatomies. He ome of the great remaining mysteries groups belong in the highlights how palaeontological, develop- in zoology concern origins — of multi- deuterostome lineage mental and genomic data now all support cellularity, complex nervous systems, of animals alongside the idea that the common ancestor of chor- Slife cycles and sex, for example. The chordates — have dates, hemichordates and echinoderms had evolutionary origin of vertebrates is among largely been resolved. pharyngeal gill slits for filter feeding. That the most intractable of these, despite more Others are as intrac- gives us a glimpse of the early chordate ances- than a century of work spanning a range of table as ever, including tor, which lived around 600 million years ago. disciplines and animal groups. where to place key fos- Other features, such as a complex brain, prob- In Across the Bridge, Henry Gee reviews sil groups such as the ably emerged much later. Having established the most recent research in this area. Gee curious vetulicolians, Across the Bridge: a hazy picture of the earliest chordates, Gee (the senior editor responsible for palae- which lived during Understanding focuses on building vertebrates and their the Origin of the ontology and evolutionary development the Cambrian period, Vertebrates defining features from the basic chordate at Nature) synthesizes contributions from some 541 million to HENRY GEE body plan, for example through spectacular anatomy, developmental biology, genomics, 485 million years ago. -

Resembling Those of Antigen Receptors Receptor Family with A
Hagfish Leukocytes Express a Paired Receptor Family with a Variable Domain Resembling Those of Antigen Receptors This information is current as Takashi Suzuki, Tadasu Shin-I, Asao Fujiyama, Yuji Kohara of September 29, 2021. and Masanori Kasahara J Immunol 2005; 174:2885-2891; ; doi: 10.4049/jimmunol.174.5.2885 http://www.jimmunol.org/content/174/5/2885 Downloaded from References This article cites 37 articles, 11 of which you can access for free at: http://www.jimmunol.org/content/174/5/2885.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 29, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2005 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Hagfish Leukocytes Express a Paired Receptor Family with a Variable Domain Resembling Those of Antigen Receptors1,2 Takashi Suzuki,* Tadasu Shin-I,§ Asao Fujiyama,†¶ʈ Yuji Kohara,‡§ and Masanori Kasahara3* Jawed vertebrates are equipped with TCR and BCR with the capacity to rearrange their V domains. -

Phylum Chordata
Phylum Chordata 48,000 species very diverse phylum but still more unity in major characteristics than in most other phyla most advanced phylum of animal kingdom one to which we belong along with fish, amphibians reptiles, birds and other mammals some of the largest or most massive animals true coelom 4 major identifying characteristics: 1. Notochord flexible rodlike structure enclosed by a fibrous sheath extends the length of the body in larva and/or adult provides basic support and serves as main axis for muscle attachments to permit “fishlike” undulatory movements first part of skeleton to form in embryo in primitive chordates the notochord persists through life Animals: Chordates & Introduction to Vertebrates; Ziser Lecture Notes, 2006 1 in most chordates the notochord is replaced by a vertebral column of bone remnants of the notochord remain as “intervertebral discs” 2. Dorsal tubular nerve cord in most invert groups; nerve cord is ventral & paired in chordates the nerve cord is a single dorsal hollow nerve cord front end usually enlarged to form brain 3. Pharyngeal (gill) slits slit-like opening sleading from throat to outside first evolved as a filter feeding apparatus still used by some to filter water for food in others as gills in some groups they are only found in embryo and lost as adults 4. endostyle or thyroid gland specific kind of tissue found only in chordates was originally part of the feeding apparatus endostyle secretes mucus and traps food inside the pharyngeal cavity eg. lamprey larva in most chordates the same tissue has become an endocrine Animals: Chordates & Introduction to Vertebrates; Ziser Lecture Notes, 2006 2 gland in the neck region that helps control metabolism 5. -

Abstract Introduction
Marine and Freshwater Miscellanea III KEY TRAITS OF AMPHIOXUS SPECIES (CEPHALOCHORDATA) AND THE GOLT1 Daniel Pauly and Elaine Chu Sea Around Us, Institute for the Oceans and Fisheries University of British Columbia, Vancouver, B.C, Canada Email: [email protected] Abstract Major biological traits of amphioxus species (Cephalochordata) are presented with emphasis on the size reached by their 32 valid species in the genera Asymmetron (2 spp.), Branchiostoma (25 spp.), and Epigonichthys (5 spp.) and on related features, i.e., growth parameters and size at first maturity. Overall, these traits combined with features of their respiration, suggest that the cephalochordates conform to the Gill Oxygen Limitation Theory (GOLT), which relates the growth performance of water-breathing ectotherms to the surface area of their respiratory organ(s). Introduction The small fish-like animals know as ‘lancelet‘ or ‘amphioxius’ belong the subphylum Cephalochordata, which is either a sister group, or related to the ancestor of the vertebrate animals (see Garcia-Fernàndez and Benito-Gutierrez 2008). The cephalochordates consist of 3 families (the Asymmetronidae, Epigonichthyidae and Branchiostomidae), with one genus each, Asymmetron (2 spp.), Branchiostoma (24 spp.) and Epigonichthys (6 spp.), as detailed in Table 1 and SeaLifeBase (www.sealifebase.org). This contribution is to assemble some of the basic biological traits of lancelets (Figure 1), notably the maximum size each of their 34 species can reach, which is easily their most important attribute, though it is often ignored (Haldane 1926). Finally, reported lengths at first maturity of cephalochordates were related to the corresponding, population-specific maximum length, to test whether these animals mature as predicted by the Gill- Oxygen Limitation Theory (GOLT; see Pauly 2021a, 2021b). -

Constraints on the Timescale of Animal Evolutionary History
Palaeontologia Electronica palaeo-electronica.org Constraints on the timescale of animal evolutionary history Michael J. Benton, Philip C.J. Donoghue, Robert J. Asher, Matt Friedman, Thomas J. Near, and Jakob Vinther ABSTRACT Dating the tree of life is a core endeavor in evolutionary biology. Rates of evolution are fundamental to nearly every evolutionary model and process. Rates need dates. There is much debate on the most appropriate and reasonable ways in which to date the tree of life, and recent work has highlighted some confusions and complexities that can be avoided. Whether phylogenetic trees are dated after they have been estab- lished, or as part of the process of tree finding, practitioners need to know which cali- brations to use. We emphasize the importance of identifying crown (not stem) fossils, levels of confidence in their attribution to the crown, current chronostratigraphic preci- sion, the primacy of the host geological formation and asymmetric confidence intervals. Here we present calibrations for 88 key nodes across the phylogeny of animals, rang- ing from the root of Metazoa to the last common ancestor of Homo sapiens. Close attention to detail is constantly required: for example, the classic bird-mammal date (base of crown Amniota) has often been given as 310-315 Ma; the 2014 international time scale indicates a minimum age of 318 Ma. Michael J. Benton. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Philip C.J. Donoghue. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Robert J. -

Lamprey, Hagfish
Agnatha - Lamprey, Kingdom: Animalia Phylum: Chordata Super Class: Agnatha Hagfish Agnatha are jawless fish. Lampreys and hagfish are in this class. Members of the agnatha class are probably the earliest vertebrates. Scientists have found fossils of agnathan species from the late Cambrian Period that occurred 500 million years ago. Members of this class of fish don't have paired fins or a stomach. Adults and larvae have a notochord. A notochord is a flexible rod-like cord of cells that provides the main support for the body of an organism during its embryonic stage. A notochord is found in all chordates. Most agnathans have a skeleton made of cartilage and seven or more paired gill pockets. They have a light sensitive pineal eye. A pineal eye is a third eye in front of the pineal gland. Fertilization of eggs takes place outside the body. The lamprey looks like an eel, but it has a jawless sucking mouth that it attaches to a fish. It is a parasite and sucks tissue and fluids out of the fish it is attached to. The lamprey's mouth has a ring of cartilage that supports it and rows of horny teeth that it uses to latch on to a fish. Lampreys are found in temperate rivers and coastal seas and can range in size from 5 to 40 inches. Lampreys begin their lives as freshwater larvae. In the larval stage, lamprey usually are found on muddy river and lake bottoms where they filter feed on microorganisms. The larval stage can last as long as seven years! At the end of the larval state, the lamprey changes into an eel- like creature that swims and usually attaches itself to a fish. -

Metabarcoding in the Abyss: Uncovering Deep-Sea Biodiversity Through Environmental
Metabarcoding in the abyss : uncovering deep-sea biodiversity through environmental DNA Miriam Isabelle Brandt To cite this version: Miriam Isabelle Brandt. Metabarcoding in the abyss : uncovering deep-sea biodiversity through environmental DNA. Agricultural sciences. Université Montpellier, 2020. English. NNT : 2020MONTG033. tel-03197842 HAL Id: tel-03197842 https://tel.archives-ouvertes.fr/tel-03197842 Submitted on 14 Apr 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THÈSE POUR OBTENIR LE GRADE DE DOCTEUR DE L’UNIVERSITÉ DE M ONTPELLIER En Sciences de l'Évolution et de la Biodiversité École doctorale GAIA Unité mixte de recherche MARBEC Pourquoi Pas les Abysses ? L’ADN environnemental pour l’étude de la biodiversité des grands fonds marins Metabarcoding in the abyss: uncovering deep - sea biodiversity through environmental DNA Présentée par Miriam Isabelle BRANDT Le 10 juillet 2020 Sous la direction de Sophie ARNAUD-HAOND et Daniela ZEPPILLI Devant le jury composé de Sofie DERYCKE, Senior researcher/Professeur rang A, ILVO, Belgique Rapporteur