MTL-CEBPA, a Small Activating RNA Therapeutic Up-Regulating C/EBP-Α
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
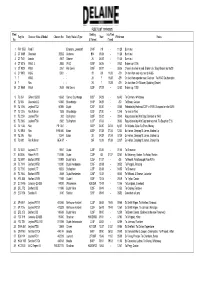
View Fleet List (1919-2021)
FLEET LIST (1919-2021) Fleet Seating Into Fleet Reg No:Chassis: Make & Model Chassis No: Body: Make & Type New Withdrawn Notes No: & Format if used 1 PW 1558 Ford T Economy, Lowestoft B14F -/19 - 11/28 Burnt out 2 CT 6489 Chevrolet 29262 Andrews B14 05/24 - 11/28 Burnt out 3 CT 7421 Lancia 4947 Delaine 26 06/25 - 11/28 Burnt out 4 CT 8216 W&G. L 2553 W&G B26F 06/26 - 09/32 Broken up 01/36 5 CT 9025 W&G 3261 Hall Lewis B26F 05/27 - 06/36 Chassis to shed in yard, Broken Up : Body Broken up 06/39 6 CY 8972 W&G 5001 - 28 -/28 11/28 -/29 On loan from and returned to W&G 7 ? W&G - - 26 ? 11/28 -/29 On loan from operator near Cadnam : To W&G Southampton 8 ? Reo - - 26 ? 11/28 -/29 On loan from CH Skinner, Spalding (Dealer) 18 CT 9869 W&G 2639 Hall Lewis B32F 07/28 - 02/52 Broken up 11/55 9 TL 364 Gilford 166SD 10668 Clarke, Scunthorpe B26F 04/29 - by/40 To Canham, Whittlesey 10 TL 565 Chevrolet LQ 54366 Bracebridge B14F 06/29 - -/30 To Brown, Caister 19 TL 1066 Leyland TS2 60895 Duple C31F 03/30 - 03/55 Rebodied by Holbrook C37F in 1939. Scrapped on site 03/55 17 TL 1316 Reo Pullman 1528 Bracebridge B20F 07/30 - 12/48 To shed in Yard 11 TL 2224 Leyland TS4 202 Burlingham B32F 03/32 - 08/40 Requistioned by War Dept. Bombed in 1943 20 TL 2965 Leyland TS6 2982 Burlingham C37F 07/33 - 06/53 Requistioned by War Dept and returned. -

Drucksache 19/27457 19
Deutscher Bundestag Drucksache 19/27457 19. Wahlperiode 10.03.2021 Antwort der Bundesregierung auf die Kleine Anfrage der Abgeordneten Torsten Herbst, Frank Sitta, Oliver Luksic, weiterer Abgeordneter und der Fraktion der FDP – Drucksache 19/26671 – Entwicklung der wirtschaftlichen Lage der Arriva PLC Vorbemerkung der Fragesteller Im Jahr 2010 hat die Deutsche Bahn AG (DB AG) für rund 2,7 Mrd. Euro in- klusive der Übernahme vorhandener Schulden das britische Unternehmen Ar- riva PLC erworben. Mit Sitz in Sunderland betreibt Arriva in mehreren euro- päischen Ländern Bus- und Bahnverkehre. Zunächst galt das Unternehmen als wirtschaftliche attraktive Akquisition für die DB AG. So erwirtschaftete Arri- va Anfang der 2010er-Jahre mit rund 50 000 Mitarbeitern einen Jahresumsatz von mehr als 5 Mrd. Euro. (https://www.handelsblatt.com/26044118.html) Vor dem Hintergrund der immer weiter steigenden Schuldenlast des DB-Konzerns unternahm der DB-Vorstand im Jahr 2019 zunächst den Versuch, Arriva zu verkaufen. Zu diesem Zeitpunkt beliefen sich die Schulden der DB AG, auch aufgrund neuer Bilanzierungsvorgaben, auf 25 Mrd. Euro. Geplant war zu- nächst, durch den Komplettverkauf bis zu vier Mrd. Euro einzunehmen. Im weiteren Verlauf des Jahres 2019 scheiterte der Verkaufsversuch jedoch. Medienberichten war zu diesem Zeitpunkt zu entnehmen, dass dem DB- Aufsichtsrat die von Investoren genannten Angebote zu niedrig gewesen sei- en. So sollte zumindest der ursprüngliche Kaufpreis von rund 3 Mrd. Euro durch den Verkauf erzielt werden (https://www.spiegel.de/wirtschaft/unterneh men/arriva-und-brexit-deutsche-bahn-blaest-boersengang-bei-britischer-tochte r-ab-a-1292544.html). Da ein solches Angebot nicht vorlag, entschied die DB AG daraufhin, Arriva im Jahr 2020 an die Börse zu bringen. -

Vehicles Expected to Attend the PEAK PARK PRESERVED BUS GATHERING 2021 As at 26Th June 2021
Vehicles expected to attend the PEAK PARK PRESERVED BUS GATHERING 2021 as at 26th June 2021 The list below shows the buses and coaches that have been entered in this year's Peak Park Preserved Bus Gathering so far. Those marked ▲ are expected to assist in operating the free bus services during the day. Additional vehicles are expected to be entered during the next few weeks - if you would like to bring a preserved bus or coach to our event, please download an entry form from our website - www.peakparkrally.wordpress.com Registration New Make Model Body Operator Livery Class A - Half cab & 'Classic' Double-Deckers ▲ WLT 467 1960 AEC Routemaster Park Royal London Transport YNU 351G 1968 Bristol FLF Eastern Coachworks Midland General ▲ OVL 473 1960 Bristol FS6G Eastern Coachworks Lincolnshire Road Car ▲ 225 LRB 1960 Leyland PD2 M.C.W. Chesterfield Transport Class B - Rear Engined Double-Deckers ▲ C253 FRJ 1986 Leyland Olympian Northern Counties Wigan Corporation ▲ P915 RYO 1997 Volvo Olympian Northern Counties London General PUM 149W 1980 Bristol VRT3LS/6LXB Eastern Coachworks West Yorkshire R.C. ▲ S629 MKH 1998 Volvo Olympian Northern Counties East Yorkshire M.S. RNA 236J 1971 Daimler Fleetline Park Royal Selnec PTE ▲ UHA 225H 1969 Daimler Fleetline Alexander Midland Red DEM 779Y 1982 Leyland Atlantean Alexander MTL Southport & Dist ▲ H654 VVV 1990 Leyland Olympian Alexander RL Stagecoach United Counties B741 GCN 1985 Leyland Olympian Eastern Coachworks Go Ahead Northern Class C - Single Deckers pre-suffix registrations (pre, 1964) MHY 765 1950 Leyland Comet Duple Orient Coaches KRR 255 1949 AEC Regal III Weymann Midland General Class D - Single Deck Buses post-suffix registrations (after 1964) ▲ BVP 808V 1980 Leyland National Leyland NBC Midland Red ▲ LED 71P 1976 Bristol RE East Lancs Warrington Transport AXI 2541 1982 Bristol RELL6G Alexander (Belfast) Citybus (Belfast) ▲ AJA 139B 1964 Bedford VAL Strachan North Western ▲ GSO 90V 1979 Leyland Leopard Alexander Y type Alexander Northern PDJ 269L 1972 AEC Swift Marshall St. -

Alphabetical Index
Alphabetical Index A & C Black PLC 3 Anglo Nordic Holdings PLC 16 Baynes, Charles, pic 30 A & J Mucklow Group PLC 3 Anglo Pacific Resources PLC 16 B.C.E. Holdings PLC 31 A B Caller & Sons Ltd 3 Ansbacher, Henry, Holdings PLC 16 BDA Holdings PLC 31 A Beckman PLC 3 Antofagasta Holdings PLC 16 B.D.R. (Grain) Ltd 31 A Cohen & Co pic 3 API Group PLC 17 BEA 39 A F Budge (Building) Ltd 3 Apollo Leisure Group PLC 17 Beale, J E, PLC 31 A F Bulgin & Co PLC 3 Apollo Metals pic 17 Beales Hunter PLC 32 A G Barr pic 3 Apollo Watch Products PLC 17 Beattie, James, PLC 32 A H Ball Group PLC 3 Appleby Westward Group pic 17 Beauford PLC 32 A H Marks & Company Ltd 3 Aquascutum Group PLC 18 Beckenham Group pic (The) 32 A R Dennis PLC 3 ARA Services Pic 18 Beckman, A, PLC 33 Abaca Group PLC (formerly Zurich Arcadian International PLC (formerly Becton Dickinson UK Ltd 33 Group PLC) 3 Westminster & Country Properties Bell & Howell Ltd 33 ABB Power Ltd 3 PLC) 18 Belling & Co Ltd 33 ABB Vetco Gray UK Ltd 3 Arco Ltd 18 Bellwinch pic 33 Abbey Panels Investments PLC 3 Arcolectric (Holdings) pic 18 Bemrose Corporation pic 33 Abbey pic 3 Arenson Group PLC 19 Bemrose, Eric, Ltd 34 Abbeycrest PLC 4 Ariston Domestic Appliances Ltd 19 Ben Bailey Construction PLC 34 ABE 22 Arlen PLC 19 Bendix Ltd 34 Aberdeen Steak Houses Group pic 4 Armitage Bros PLC 19 Bennett & Fountain Group pic 34 Aberfoyle Holdings PLC 4 Armour Trust pic 19 Benson Group PLC 34 ABI Leisure Group PLC 4 Arsenal Football Club PLC 20 Bensons Crisps PLC 34 Abrams, Syd, Ltd 5 Arthur Sanderson & Sons Ltd 20 -

Annual Bus Statistics: 2010/11
Annual Bus Statistics: 2010/11 Notes and Definitions This document provides information about DfT These Notes and Definitions include: bus statistics. 1. Introduction to the statistics Bus statistics are published annually by the 2. Information on data sources and methods Department for Transport, and include figures 3. Information relating to the published tables relating to bus passenger journeys, vehicle miles including key definitions travelled, revenue and costs, fare levels, 4. Sources of further information related to but Government support, vehicles owned by PSV not covered by these statistics operators and number of staff employed. In 5. Background and contextual information addition to the annual publication, estimates of patronage are available on a quarterly basis. Section 1 presents a brief overview of the statistics, covering the following questions: What do these statistics cover? Why are the statistics collected and how are they used? What are the sources of data used to compile the statistics? What methods are used to compile the published information? How reliable are the statistics? What should be considered when using them? How often are these statistics updated? What other information is available on buses and bus travel? Section 2 provides further general information about the main data sources used to compile the statistics, including the methods used to produce figures for publication and data quality issues. Section 3 presents information relevant to specific aspects of the published figures, including definitions of key terms and specific issues relevant to the interpretation of individual tables or sections. Section 4 provides details of further sources of statistics on buses and bus travel which are not covered by these statistics Section 5 provides links to relevant contextual information about the bus industry and bus policy The annexes contain more detailed information as referenced in the appropriate section above. -

Greater London News Sheet 850-1-285 November 2010
Please send your reports, observations, and comments by Mail to: The PSV Circle, Unit 1R, Leroy House, 1 436 Essex Road, LONDON, N1 3QP by FAX to: 0870 051 9442 by email to: [email protected] GREATER LONDON NEWS SHEET 850-1-285 NOVEMBER 2010 MAJOR OPERATORS ABELLIO London Limited {abellio london} (LN) / ABELLIO West London Limited {abellio london / abellio surrey} (Abellio) Opening Fleet (1/6/10) updated information 8058 (Y864 KTF ex 1068 MW) transferred from Travel London (LN) 8058 Corrections 849-1-269 delete vehicle out 8005 (Y215 HWF) - (still in use 10/10). Allocations by9/10: 8478-87.TF. Vehicles out (Note all these are deleted from stock, but may well remain on depots awaiting collection by the lessors). 8006 (Y116 HWB), 8007 (Y117 HWB), 8008 (Y118 HWB), 8010 (Y 42 HVV), 8021 (BU 05 HDO), 8022 (BU 05 HDV): gone c9/10 8023 (BU 05 HDX): Ensign, Purfeet (Q) 9/10 8030 (BU 05 HFG), 8032 (BU 05 HFM), 8033 (BU 05 FFN), 8034 (BU 05 HFT), 8037 (BU 05 HFX), 8038 (BU 05 HFY), 8039 (BU 05 HFZ), 8075 (KN 52 NFO), 8076 (KN 52 NFP), 8077 (KN 52 NFR), 8078 (KN 52 NFT), 8079 (KN 52 NFU), 8080 (KN 52 NFV), 8081 (KN 52 NFX), 8082 (KN 52 NFY), 8083 (KN 52 NFC), 8084 (KN 52 NFD), 8085 (KN 52 NFE), 8096 (YT 51 DZZ), 8097 (YT 51 EAA), 8098 (YT 51 EAJ), 8099 (YT 51 EAP), 8401 (W401 UGM), 8402 (W402 UGM), 8403 (W403 UGM), 8404 (W404 UGM), 8407 (W407 UGM), 8408 (W408 UGM), 8409 (W409 UGM), 8411 (W411 UGM), 8412 (W412 UGM), 8413 (W413 UGM), 8721 (W601 UGM), 8722 (W602 UGM), 8723 (W603 UGM), 8724 (W604 UGM), 8725 (W605 UGM), 8726 (W606 UGM), 8727 (W607 UGM), 8728 (W608 UGM), 8729 (W609 UGM), 8731 (W611 UGM), 8732 (W612 UGM), 8841 (YT 51 EAW), 8842 (YT 51 EAX), 8843 (YT 51 EAY), 8844 (YT 51 EBA): gone c9/10. -

Post-Marketplace Version
AMERICAN BUS MARKETPLACE 2017 DIRECTORY OF PARTICIPANTS Cleveland, OH Jan. 14-17, 2017 111 K Street NE, 9th Fl. • Washington, DC 20002 (800) 283-2877 (U.S & Canada) • (202) 842-1645 • Fax (202) 842-0850 [email protected] • www.buses.org This Directory includes Buyers, Sellers and Associate delegates. It does not include Operators attending Marketplace as other registration types. Directory as of Jan. 17, 2017 To find more information about the companies and delegates in this publication, please click on the Research Database stamp in your Marketplace Passport, ABA Marketplace 2017 App or visit My ABA. Section I MOTORCOACH AND TOUR OPERATOR BUYERS Motorcoach & Tour Operators (Buyers) page 3 AT&T Charter Service Inc. www.corporatecoachesfla.com AAA East Central 10 Jilden Dr. Mike Castro, V.P. Business Dev. 5900 Baum Blvd. Louisburg, NC 27549 [email protected] Pittsburgh, PA 15206 www.attcharterservice.com www.aaa.com Kendra Love Academy Bus LLC Claudia Zuzak 111 Patterson Ave. [email protected] Hoboken, NJ 07030-6012 Marlene Santini A Yankee Line Inc [email protected]> 15 County Ave. www.academybus.com Myrna Blanda, Gp. Sales Coord. Secaucus, NJ 07094 Dave Bolen [email protected] [email protected] Frank Smith Rose Marie Bakos. Francis Tedesco, Pres. [email protected] [email protected] AAA Ohio Auto Club Stephanie Ventura, Gp. Trvl. Coord. for Gregory Tedesco, Sales and Marketing NEO 90 E. Wilson Bridge Rd. [email protected] [email protected] Worthington, OH 43085 Michael Coyle, Safety Mgr. www.aaa.com [email protected] Michael Horak, Dir., Operational Risk AAA Northwest Ohio Beth Mowry, Group Travel Coordinator [email protected] Mgmt. -

Turn to and CONSTRUCTION RECORD Î Daily Commercial News News Daily Commercial Page 2 Daily Commercial News November 20, 2018
GDP Read online Alex Carrick dcnonl.com/news/economic Daily Commercial News by Construct Connect® NOVEMBER 20, 2018 www.dailycommercialnews.com VOL. 91 NO. 224 $5.23 plus HST ALERT! Smart cities need trust, communication to succeed: panel In the event of a Canada Post strike, WARREN FREY you will have access to a digital copy implement technological solutions. DIGITAL MEDIA EDITOR of the Daily Commercial News: CIBC Square Rises “It’s much easier to buy Zambo- www.dailycommercialnews.com/ mart cities are brimming nis and photocopiers for a munici- issue with potential but come pality than machine learning soft- with caveats. ware,” Robinson said. If you have any questions please S A group of smart city experts Gunnar Edwin Crawford, the contact our Customer Relations team spoke at a panel during the Cana- head of the city of Stavanger, Nor- at 1-800-959-0502. dian Council for Public-Private way’s smart city project, said the Partnerships recent national con- biggest challenge in creating a ference held in Toronto. road map document involving The group picked apart the chal- academia, the private sector and In Brief lenges and potential of “upgrading” other participants was a lack of a city from both a technological communication. Governments announce and societal standpoint. “It’s hard. You have all these One of the major challenges experts, but they don’t talk to one new Inuvik wind project of building a smart city or com- another,” he said, adding he “spent INUVIK, N.W.T. munity is defining it, said World 70 per cent of the time trying to Representatives from three levels of Bank senior director of social, break down barriers.” government have announced $40 mil- urban, rural and resilience global Crawford said opening data lion in funding for the Inuvik Wind practice Ede Ijjasz-Vasquez. -

A Directory of Regularly Scheduled, Fixed Route, Local Public
A DIRECTORY OF REGULARLY SCHEDULED, FIXED ROUTE, LOCAL PUBLIC TRANSPORTATION SERVICE IN URBANIZED AREAS OVER 50,000 POPULATION AUGUST 1981 U.S. DEPARTMENT OF TRANSPORTATION Urban Mass Transportation Administration Office of Planning, Management and Demonstrations Washington, D.C. 20590 . FOR RELEASE MONDAY UMTA Technical Notice 2-81 August 10, 1981 The U. S. DeDartment of Transportation's Urban Mass Transportation Administration released today an updated directory of 686 local fixed route transit operations in 279 urbanized areas (UZAs) of over 50,000 population. As shown in the summaries on page 19, the vehicle requirements for weekday schedules in these UZAs are 3,566 commuter rail cars, 7,450 rapid transit cars, 551 light rail cars, 42,925 motor coaches, 503 trolley coaches and 28 cable cars. These figures are peak requirements, not fleet size. For purposes of this compilation, a transit operation is a fixed route, regularly scheduled service, available to the general public, offering rides wholly within, or commuter rides from outside a particular UZA. Maps showing UZA borders appear in U.S. Census Bureau publication PC (1)-A, by states. UZA listings are in the order of population rank. To find a particular UZA, refer to page 1, where UZAs are listed alphabetically, or to page 21, where they are listed by states. Users of this directory desiring to identify systems of a certain size, or operating different modes, will find useful information in column 5. This directory replaces Technical Notice 2-80 dated August 1, 1 980. Another listing in this series is TN 1-81 dated March 9, 1981, reporting services available in communities of less than 50 ,000 population. -

Passenger Rail Franchising – British Experience
PASSENGER RAIL FRANCHISING – BRITISH EXPERIENCE Chris Nash Andrew Smith (ITS, University of Leeds)* ABSTRACT Given that virtually all British passenger train services were franchised out over the period 1995-7, and many have now been franchised for a second time, Britain should provide an excellent opportunity to study the impact of franchising passenger rail services. Moreover, since several different franchising models have been tried, there should also be some useful evidence on how best to go about franchising. In practice, however, the turbulent history of the British rail industry over this period makes drawing firm conclusions difficult. At the start, it appeared that franchising was very successful with strong competition for franchises, rapidly rising traffic, rising productivity and falling subsidies. Whilst most of the increase in traffic was due to external factors, the growth appears somewhat faster than would be explained by these factors alone. Despite this, a number of train operating companies got into financial difficulties, particularly in the Regional sector, where franchisees were relying on reduced costs rather than increased revenues to achieve subsidy reductions, and in the short term franchises were renegotiated or replaced with cost-plus contracts pending refranchising. After the bankruptcy of Railtrack not only have the costs and performance of the infrastructure manager severely deteriorated, but there has also been a large rise in the costs of train operating companies. Without a better understanding of the causes of this rise it is hard to form firm conclusions on the success of franchising. One argument is that one of the reasons franchisees found it difficult to achieve the anticipated cost reductions was the degree to which costs had already been driven down in the 1980s. -

27. Group Undertakings
Notes to the accounts (continued) 26. Acquisitions 27. Group undertakings In November 2009 the group acquired the remaining 20 per cent of the share capital of Eurobus Invest for £0.4 million. Detailed below is a list of those subsidiaries which in the opinion of the directors principally affect the amount of the profit or the amount of the assets of the group. The group percentage of equity capital is 100 per cent and the country of registration is England Prior year acquisitions: and Wales in each case, except where indicated. All subsidiaries operate within England and Wales, except where indicated: Adjustments in respect of prior year acquisitions are detailed in the table below: £m Passenger Transport Rental and Distribution of Buses and Coaches Hindsight period adjustments: Arriva Cymru Limited Arriva Bus and Coach Rental (4) Limited Trade and other receivables 0.1 Arriva Derby Limited Arriva Bus and Coach Limited Trade and other payables 1.4 Arriva Durham County Limited Corporation tax (0.1) Arriva East Herts & Essex Limited Investment Arriva International Trains (Leasing) Limited Deferred tax (0.2) Arriva Findiv Limited Arriva Kent & Sussex Limited Arriva International Limited* Decrease in fair values 1.2 Arriva Kent Thameside Limited Arriva Motor Holdings Limited* Consideration received (0.7) Arriva London North Limited Arriva Passenger Services Limited* Arriva London South Limited 0.5 MTL Services Limited* Arriva Medway Towns Limited Arriva Insurance Company (Gibraltar) Limited*12 Arriva Merseyside Limited Goodwill based on provisional fair values 50.1 Arriva International (Northern Europe) Limited Arriva Midlands Limited Arriva International (Southern Europe) Limited Arriva Midlands North Limited Goodwill based on final fair values 50.6 Arriva Malta Holdings Limited Arriva Noroeste SL2 Arriva (2007) Limited Arriva Northumbria Limited Arriva North West Limited Comparative amounts have not been restated following the final determination of fair value adjustments as the amounts involved Property are not material. -
RELEASES ISSUE 10 2008 1:76 Scale/00 Gauge Precision Diecast Models
GI1-Issue 10 2008.qxd 24/10/08 16:24 Page 1 EW N RELEASES ISSUE 10 2008 1:76 Scale/00 gauge precision diecast models Details May Be Subject to Change NEW TOOLING 31002 Foden S24 Four Axle Flatbed D.A.MACRAE 36201 RMA Routemaster and Trailer BRITISH EUROPEAN AIRWAYS Having received many requests for more Foden S24 lorries. Our second release features The Airport Routemasters with their “Customs” sealed trailers operated a unique service a four axle flatbed operated by D.A. Macrae of Fraserburgh and Hull. This was used to allowing travellers to check in for Heathrow flights at the central London Terminal in transport their red ‘M’ brand Kippers. NOVEMBER RELEASE. Kensington. BEA 41, registered NMY 641E, pulls trailer number N 150 to Heathrow where the BEA aircraft awaited its arrival. This model takes us back to a more civilized era of air travel. NOVEMBER RELEASE. 23319 AEC MkI RF Bus LONDON TRANSPORT We were recently reminded, much to our surprise that we had only previously released 35002 AEC Mercury Articulated Flatbed BARTON TRANSPORT one AEC RF MkI bus in London Transport Country colours and that was the very first release. Registered NLE 584, fleet number RF584 operates route 432 to Great Bookham, Continuing to build our Barton Transport fleet this is the first release of our AEC Mercury this ever popular bus is sure to prove highly collectable. DECEMBER RELEASE. with an articulated flatbed. Registered 626 KNN, fleet number 71 carries a crate and sheeted backload. NOVEMBER RELEASE. 20641 Plaxton Pointer Dart MTL LONDON This MTL London Plaxton Pointer Dart is the first of a new fleet for our models.