Supporting Information a Analysed Substances
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Drug Class Review Beta Adrenergic Blockers
Drug Class Review Beta Adrenergic Blockers Final Report Update 4 July 2009 Update 3: September 2007 Update 2: May 2005 Update 1: September 2004 Original Report: September 2003 The literature on this topic is scanned periodically. The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use, or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Mark Helfand, MD, MPH Kim Peterson, MS Vivian Christensen, PhD Tracy Dana, MLS Sujata Thakurta, MPA:HA Drug Effectiveness Review Project Marian McDonagh, PharmD, Principal Investigator Oregon Evidence-based Practice Center Mark Helfand, MD, MPH, Director Oregon Health & Science University Copyright © 2009 by Oregon Health & Science University Portland, Oregon 97239. All rights reserved. Final Report Update 4 Drug Effectiveness Review Project TABLE OF CONTENTS INTRODUCTION .......................................................................................................................... 6 Purpose and Limitations of Evidence Reports........................................................................................ 8 Scope and Key Questions .................................................................................................................... 10 METHODS................................................................................................................................. -

Advice for Primary Care Regarding Beta Blockers in Heart Failure and Qof
Advice for Primary Care Regarding Beta-Blockers in Heart Failure and QOF ADVICE FOR PRIMARY CARE REGARDING BETA BLOCKERS IN HEART FAILURE AND QOF Author(s): Trudi Phillips, Lead Nurse, SEWCN / Heart Failure Specialist Nurse, Cwm Taf HB Date: 20 th May 2010 Version: 4: Status Final Pathway: Heart Failure Intended Audience: Cardiac Network, GPs and Primary Care Staff Purpose and Summary of Document: To advise GPs and primary care on the initiation of Beta Blockers for patients with heart failure and changing Beta Blocker medication. Publication / Distribution: • Cardiac Network primary care distribution list • Cardiac Network GPs via email • Network website ( http://www.sewcn.wales.nhs.uk ; http://nww.sewcn.wales.nhs.uk ) • Highlight in next e-Newsletter and Heart Matters Date of Issue: 21 st May 2010 Review Date: May 2011 Date published on Network Website: 10 th May 2010 South East Wales Cardiac Network Advice for Primary Care Regarding Beta-Blockers in Heart Failure and QOF Author: Trudi Phillips. SEWCN and Cwm Taf Date: 7th May 2010 Status: Final HB Intended Audience: Page: 1 of 3 Cardiac Network GPs Primary Care Staff Advice for primary care regarding Beta-blockers in Heart Failure and QOF Carvedilol, Bisoprolol and Nebivolol (in the elderly) are the only three beta-blockers currently licensed for use in heart failure in the UK. Beta-blockade therapy for heart failure should be introduced in a ‘ start low, go slow ’ manner, with assessment of heart rate, blood pressure, and clinical status after each titration. Beta blocker Starting dose Maximum target dose Bisoprolol 1.25 mg od 10 mg od Carvedilol 3.125 mg bd 25mg bd Nebivolol (in the elderly) 1.25 mg od 10 mg od For patients with mild to moderate heart failure maximum dose of Carvedilol is 50 mg twice daily if weight more than 85 kg How to use: • Start with a low dose (see above). -

Allergy Testing and History Form
Skin Testing Information and Consent 1. Skin Testing An allergy skin test is used to identify the substances that are causing your allergy symptoms. We will apply several extracts of common allergens to the skin and observe for a reaction. The reactions are then graded and confirmatory intradermal testing may be performed. This involves placing a small amount of extract under the skin of the upper arm. We then observe the reaction and record the results. On the day of testing please wear a short-sleeved shirt that can be pushed up comfortably to your shoulder. Allow 1-2 hours for your test session. You will need to stay on the premises during this time. Please do not bring children to your appointment. 2. Risks of Skin Testing Bleeding and infection may occur due to abrading of the skin. Any time the skin integrity is broken it puts on at risk for infection. However, this is a rare occurrence. The antigens used for testing are sterile and approved by the FDA. Occasionally, skin testing can trigger a severe allergic reaction requiring treatment with medications and/or treatment in the ER. Patients with asthma are at increased risk for triggering an asthma attack during testing. You should not undergo testing if you feel that you have allergy or asthma symptoms are currently under poor control. 3. Contraindications to Skin Testing Women who are pregnant or anyone who is currently taking Beta-Blockers, Tricyclic Anti-depressants or MAOI’s medications will NOT be skin tested. Please be sure to inform us of ALL your medications before the skin test is applied. -

M2021: Pharmacogenetic Testing
Pharmacogenetic Testing Policy Number: AHS – M2021 – Pharmacogenetic Prior Policy Name and Number, as applicable: Testing • M2021 – Cytochrome P450 Initial Presentation Date: 06/16/2021 Revision Date: N/A I. Policy Description Pharmacogenetics is defined as the study of variability in drug response due to heredity (Nebert, 1999). Cytochrome (CYP) P450 enzymes are a class of enzymes essential in the synthesis and breakdown metabolism of various molecules and chemicals. Found primarily in the liver, these enzymes are also essential for the metabolism of many medications. CYP P450 are essential to produce many biochemical building blocks, such as cholesterol, fatty acids, and bile acids. Additional cytochrome P450 are involved in the metabolism of drugs, carcinogens, and internal substances, such as toxins formed within cells. Mutations in CYP P450 genes can result in the inability to properly metabolize medications and other substances, leading to increased levels of toxic substances in the body. Approximately 58 CYP genes are in humans (Bains, 2013; Tantisira & Weiss, 2019). Thiopurine methyltransferase (TPMT) is an enzyme that methylates azathioprine, mercaptopurine and thioguanine into active thioguanine nucleotide metabolites. Azathioprine and mercaptopurine are used for treatment of nonmalignant immunologic disorders; mercaptopurine is used for treatment of lymphoid malignancies; and thioguanine is used for treatment of myeloid leukemias (Relling et al., 2011). Dihydropyrimidine dehydrogenase (DPD), encoded by the gene DPYD, is a rate-limiting enzyme responsible for fluoropyrimidine catabolism. The fluoropyrimidines (5-fluorouracil and capecitabine) are drugs used in the treatment of solid tumors, such as colorectal, breast, and aerodigestive tract tumors (Amstutz et al., 2018). A variety of cell surface proteins, such as antigen-presenting molecules and other proteins, are encoded by the human leukocyte antigen genes (HLAs). -

Summary of Product Characteristics
Health Products Regulatory Authority Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Salbutamol CFC-Free Inhaler 100 micrograms per metered dose, pressurised inhalation, suspension 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One metered dose contains 100 micrograms of salbutamol (equivalent to 120 micrograms of salbutamol sulphate). This is equivalent to a delivered dose of 90 micrograms of salbutamol (equivalent to 108 micrograms of salbutamol sulphate). For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Pressurised inhalation suspension Pressurised inhalation suspension supplied in an aluminium canister with a metering valve and a plastic actuator and dust cap. 4 CLINICAL PARTICULARS 4.1 Therapeutic Indications Salbutamol CFC-Free Inhaler is indicated in adults, adolescents and children. For babies and children under 4 years of age, see sections 4.2 and 5.1. Salbutamol CFC-Free Inhaler is indicated for the relief and prevention of bronchial asthma and conditions associated with reversible airways obstruction. Salbutamol CFC-Free Inhaler can be used as relief medication in the management of mild, moderate or severe asthma, provided that its use does not delay the introduction and use of regular inhaled corticosteroid therapy, where necessary. 4.2 Posology and method of administration Salbutamol CFC-Free Inhaler is for oral inhalation use only. Posology Adults (including the elderly) and adolescents (children 12 years and over): For the relief of acute bronchospasm, one inhalation (100 micrograms) increasing to two inhalations (200 micrograms), if necessary. To prevent allergen- or exercise-induced symptoms, two inhalations (200 micrograms) should be taken 10-15 minutes before challenge. Maximum daily dose: two inhalations (200 micrograms) up to four times a day. -

Different Beta-Blocking Effects of Carvedilol and Bisoprolol in Humans
Journal of Clinical and Basic Cardiology An Independent International Scientific Journal Journal of Clinical and Basic Cardiology 2001; 4 (1), 53-56 Different beta-blocking effects of carvedilol and bisoprolol in humans Koshucharova G, Klein W, Lercher P, Maier R, Stepan V Stoschitzky K, Zweiker R Homepage: www.kup.at/jcbc Online Data Base Search for Authors and Keywords Indexed in Chemical Abstracts EMBASE/Excerpta Medica Krause & Pachernegg GmbH · VERLAG für MEDIZIN und WIRTSCHAFT · A-3003 Gablitz/Austria ORIGINAL PAPERS, CLINICAL CARDIOLOGY Different Beta-Blocking Effects of Carvedilol and Bisoprolol J Clin Basic Cardiol 2001; 4: 53 Different Beta-Blocking Effects of Carvedilol and Bisoprolol in Humans G. Koshucharova, R. Zweiker, R. Maier, P. Lercher, V. Stepan, W. Klein, K. Stoschitzky Bisoprolol is a beta1-selective beta-adrenergic antagonist while carvedilol is a non-selective beta-blocker with additional blockade of alpha1-adrenoceptors. Administration of bisoprolol has been shown to cause up-regulation of β-adrenoceptor density and to decrease nocturnal melatonin release, whereas carvedilol lacks these typical effects of beta-blocking drugs. The objective of the present study was to investigate beta-blocking effects of bisoprolol and carvedilol in healthy subjects. We compared the effects of single oral doses of clinically recommended amounts of bisoprolol (2.5, 5 and 10 mg) and carvedilol (25, 50 and 100 mg) to those of placebo in a randomised, double-blind, cross-over study in 12 healthy male volun- teers. Three hours after oral administration of the drugs heart rate and blood pressure were measured at rest, after 10 min. of exercise, and after 15 min. -

Prodrugs Design Based on Inter- and Intramolecular Chemical Processes
Chem Biol Drug Des 2013; 82: 643–668 Review Prodrugs Design Based on Inter- and Intramolecular Chemical Processes Rafik Karaman1,2,* compound satisfies a number of preset criteria to start clinical development. The number of years it takes to intro- 1Bioorganic Chemistry Department, Faculty of Pharmacy, duce a drug to the pharmaceutical market is over 10 years Al-Quds University, P.O. Box 20002, Jerusalem, Palestine with a cost of more than $1 billion dollars (1,2). 2Department of Science, University of Basilicata, Via dell’Ateneo Lucano 10, 85100, Potenza, Italy Modifying the absorption, distribution, metabolism, and *Corresponding author: Rafik Karaman, elimination (ADME) properties of an active drug requires a [email protected] complete understanding of the physicochemical and bio- logical behavior of the drug candidate (3 6). This includes This review provides the reader a concise overview of – the majority of prodrug approaches with the emphasis comprehensive evaluation of drug-likeness involving on the modern approaches to prodrug design. The prediction of ADME properties. These predictions can be chemical approach catalyzed by metabolic enzymes attempted at several levels: in vitro–in vivo using data which is considered as widely used among all other obtained from tissue or recombinant material from human approaches to minimize the undesirable drug physico- and preclinical species, and in silico or computational pre- chemical properties is discussed. Part of this review dictions projecting in vitro or in vivo data, involving the will shed light on the use of molecular orbital methods evaluation of various ADME properties, using computa- such as DFT, semiempirical and ab initio for the design tional approaches such as quantitative structure activity of novel prodrugs. -

The 2013 "Research on Drug Evidence"
The 2013 “Research on Drug Evidence” Report [From the 17th ICPO / INTERPOL Forensic Science Symposium] Robert F. X. Klein U.S. Department of Justice Drug Enforcement Administration Special Testing and Research Laboratory 22624 Dulles Summit Court Dulles, VA 20166 [[email protected]] ABSTRACT: A reprint of the 2013 “Research on Drug Evidence” Report (a review) is provided. KEYWORDS: INTERPOL, Illicit Drugs, Controlled Substances, Forensic Chemistry. Important Information: Distributed at the 17th ICPO / INTERPOL Forensic Science Symposium, Lyon, France, October 8 - 10, 2013.* Authorized Reprint. Copyright INTERPOL. All rights reserved. May not be reprinted without express permission from INTERPOL. For pertinent background, see: Klein RFX. ICPO / INTERPOL Forensic Science Symposia, 1995 - 2016. “Research on Drug Evidence”. Prefacing Remarks (and a Request for Information). Microgram Journal 2016;13(1-4):1-3. Citations in this report from the Journal of the Clandestine Laboratory Investigating Chemists Association were (and remain) Law Enforcement Restricted. The "General Overview" (Talking Paper) was removed from this reprint (Editor's discretion). This reprint is derived from the original electronic document, and is not an image of the best available hard copy (as was utilized for the 1995 and 1998 reports). For this reason, the pagination in the Proceedings is not retained in this reprint, some minor reformatting was done to eliminate deadspace, and all widow and orphan lines were left as is. [* Due to travel restrictions in effect in late 2013, this report and the associated "General Overview" (Talking Paper) was not actually presented, but rather the report was only distributed to the attendees.] Microgram Journal 2016, Volume 13; Numbers 1-4 511 Research on Drug Evidence January 1, 2010 - June 30, 2013 Presented by: Jeffrey H. -

Annex 2B Tariff Schedule of the United States See General Notes to Annex 2B for Staging Explanation HTSUS No
Annex 2B Tariff Schedule of the United States See General Notes to Annex 2B for Staging Explanation HTSUS No. Description Base Rate Staging 0101 Live horses, asses, mules and hinnies: 0101.10.00 -Purebred breeding animals Free E 0101.90 -Other: 0101.90.10 --Horses Free E 0101.90.20 --Asses 6.8% B --Mules and hinnies: 0101.90.30 ---Imported for immediate slaughter Free E 0101.90.40 ---Other 4.5% A 0102 Live bovine animals: 0102.10.00 -Purebred breeding animals Free E 0102.90 -Other: 0102.90.20 --Cows imported specially for dairy purposes Free E 0102.90.40 --Other 1 cent/kg A 0103 Live swine: 0103.10.00 -Purebred breeding animals Free E -Other: 0103.91.00 --Weighing less than 50 kg each Free E 0103.92.00 --Weighing 50 kg or more each Free E 0104 Live sheep and goats: 0104.10.00 -Sheep Free E 0104.20.00 -Goats 68 cents/head A 0105 Live poultry of the following kinds: Chickens, ducks, geese, turkeys and guineas: -Weighing not more than 185 g: 0105.11.00 --Chickens 0.9 cents each A 0105.12.00 --Turkeys 0.9 cents each A 0105.19.00 --Other 0.9 cents each A -Other: 0105.92.00 --Chickens, weighing not more than 2,000 g 2 cents/kg A 0105.93.00 --Chickens, weighing more than 2,000 g 2 cents/kg A 0105.99.00 --Other 2 cents/kg A 0106 Other live animals: -Mammals: 0106.11.00 --Primates Free E 0106.12.00 --Whales, dolphins and porpoises (mammals of the order Cetacea); manatees and dugongs (mammals of the order Sirenia) Free E 0106.19 --Other: 2B-Schedule-1 HTSUS No. -

Drugs-Biologicals FORMULARY for INTERNET PAGE 12 26 18
AVG ITEM Average Patient Item Charge Code Description BIL AWP /pkg COST Price 25000041 SODIUM and POTASSIUM BICARBONATE TBEF UD $3.61 $0.12 $1.00 25000054 GLIMEPIRIDE TAB 1 MG UD $8.25 $0.11 $1.30 25000086 amLODIPine TAB 5 MG UD $8.65 $0.06 $1.00 25000087 AMMONIA AROMATIC SOLN 15 % (W/V) UD $3.53 $0.17 $1.00 25000090 AMOXICILLIN CAP 500 MG UD $20.12 $0.19 $1.00 25000102 ABACAVIR TAB 300 MG UD $507.10 $8.45 $16.90 25000103 ACAMPROSATE TBEC 333 MG UD $182.38 $0.80 $1.76 25000104 ACARBOSE TAB 25 MG UD $16.70 $0.27 $1.00 25000106 ACETAMINOPHEN SUPP 120 MG UD $5.09 $0.42 $1.00 25000108 ACETAMINOPHEN SUPP 325 MG UD $4.91 $0.44 $1.00 25000109 ACETAMINOPHEN TAB 325 MG UD $12.39 $0.02 $1.00 25000111 ACETAMINOPHEN TAB 500 MG UD $2.34 $0.02 $1.00 25000112 ACETAMINOPHEN SUPP 650 MG UD $3.83 $0.17 $1.00 25000113 ACETAMINOPHEN SOLN 650 MG/20.3 ML UD $75.74 $0.48 $1.22 25000117 ACETAMINOPHEN-CODEINE TAB 300-30 MG UD $12.41 $0.09 $1.00 25000121 ACETYLCYSTEINE SOLN 200 MG/ML (20 %) UD $18.18 $7.66 $15.32 25000140 ALBUTEROL SULFATE NEBU 2.5MG/3ML (0.083 %) UD $8.58 $0.29 $1.00 25000147 ALLOPURINOL TAB 100 MG UD $18.45 $0.12 $1.00 25000150 ALPRAZolam TAB 0.25 MG UD $8.87 $0.09 $1.00 25000151 ALPRAZolam TAB 0.5 MG UD $4.16 $0.04 $1.00 25000182 AMIODARONE TAB 200 MG UD $15.70 $0.20 $1.00 25000184 AMITRIPTYLINE TAB 10 MG UD $8.73 $0.06 $1.00 25000186 AMITRIPTYLINE TAB 25 MG UD $17.46 $0.08 $1.00 25000188 AMITRIPTYLINE TAB 50 MG UD $34.90 $0.16 $1.00 25000205 HYDROCORTISONE ACETATE SUPP 25 MG UD $127.30 $5.75 $11.50 25000206 HEMORRHOIDAL SUPPOSITORY SUPP 0.25 -

Immunologic Adverse Reactions of Β-Blockers and the Skin (Review)
EXPERIMENTAL AND THERAPEUTIC MEDICINE 18: 955-959, 2019 Immunologic adverse reactions of β-blockers and the skin (Review) ALIN LAURENTIU TATU1, ALINA MIHAELA ELISEI1, VALENTIN CHIONCEL2, MAGDALENA MIULESCU3 and LAWRENCE CHUKWUDI NWABUDIKE4 1Medical and Pharmaceutical Research Unit/Competitive, Interdisciplinary Research Integrated Platform ‘Dunărea de Jos’, ReForm-UDJG; Research Centre in the Field of Medical and Pharmaceutical Sciences, Faculty of Medicine and Pharmacy, Department of Pharmaceutical Sciences, ‘Dunărea de Jos’ University of Galați, 800010 Galati; 2Department of Cardio-Thoracic Pathology, Faculty of Medicine, ‘Carol Davila’ University of Medicine and Phamacy, 050474 Bucharest; 3Department of Morphological and Functional Sciences, Faculty of Medicine and Pharmacy, ‘Dunarea de Jos University’ of Galati, 800010 Galati; 4Department of Diabetic Foot Care, ‘Prof. N. Paulescu’ National Institute of Diabetes, 011233 Bucharest, Romania Received September 11, 2018; Accepted November 16, 2018 DOI: 10.3892/etm.2019.7504 Abstract. β-Blockers are a widely utilised class of medica- use, as well as possible therapeutic approaches to these. This tion. They have been in use for a variety of systemic disorders short review will focus on those dermatoses resulting from including hypertension, heart failure and intention tremors. β-blocker use, which have an immunologic basis. Their use in dermatology has garnered growing interest with the discovery of their therapeutic effects in the treatment of haemangiomas, their potential positive effects in wound Contents healing, Kaposi sarcoma, melanoma and pyogenic granuloma, and, more recently, pemphigus. Since β-blockers are deployed 1. Introduction in a variety of disorders, which have cutaneous co-morbidities 2. Cutaneous side - effects of β-blockers such as psoriasis, their pertinence to dermatologists cannot be 3. -
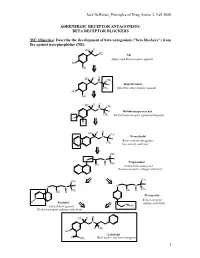
BETA RECEPTOR BLOCKERS MC Objective
Jack DeRuiter, Principles of Drug Action 2, Fall 2000 ADRENERGIC RECEPTOR ANTAGONISTS: BETA RECEPTOR BLOCKERS MC Objective: Describe the development of beta antagonists ("beta blockers") from the agonist norepinephrine (NE): HO H NH2 NE Alpha- and Beta-receptor agonist HO OH H H HO CH N 3 H Isoproterenol CH3 Selective beta-receptor agonist HO OH H H HO CH N 3 H Dichloroisoproterenol CH3 Partial beta-receptor agonist/antagonist Cl Cl H H HO CH N 3 Pronethalol H Beta-receptor antagonist, CH 3 low activity and toxic CH3 O N H Propranolol CH3 OH H Potent beta-antagonist No beta-receptor subtype selectivity CH3 CH3 O N H O N H CH CH OH H 3 OH H 3 Metoprolol N Beta-1-receptor H Pindolol O subtype selectivity Partial beta-agonist CH3 No beta-receptor subtype selectivity HO H H N H CH3 HO Labetolol O NH2 Dual alpha- and beta-antagonst 1 Jack DeRuiter, Principles of Drug Action 2, Fall 2000 MC Objective: Based on their structures, would the beta-blockers be expected to be relatively receptor selective? YES. They do not produce significant blockade of alpha- adrenergic receptors (alpha-1 or alpha-2), histamine receptors, muscarinic receptors or dopamine receptors. MC/PC Objective: Identify which beta blockers are classified as "non-selective": · The “non-selective" classification refers to those beta-blockers capable of blocking BOTH beta-1 and beta-2 receptors with equivalent efficacy. These drugs DO NOT have clinically significant affinity for other neurotransmitter receptors (alpha, dopamine, histamine, acetylcholine, etc.). · ALL of these beta-blockers (except satolol) consist of an aryloxypropanolamine side chain linked to an aromatic or “heteroaromatic” ring which is “ortho” substituted.