San Diego ENT
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Allergy Testing and History Form
Skin Testing Information and Consent 1. Skin Testing An allergy skin test is used to identify the substances that are causing your allergy symptoms. We will apply several extracts of common allergens to the skin and observe for a reaction. The reactions are then graded and confirmatory intradermal testing may be performed. This involves placing a small amount of extract under the skin of the upper arm. We then observe the reaction and record the results. On the day of testing please wear a short-sleeved shirt that can be pushed up comfortably to your shoulder. Allow 1-2 hours for your test session. You will need to stay on the premises during this time. Please do not bring children to your appointment. 2. Risks of Skin Testing Bleeding and infection may occur due to abrading of the skin. Any time the skin integrity is broken it puts on at risk for infection. However, this is a rare occurrence. The antigens used for testing are sterile and approved by the FDA. Occasionally, skin testing can trigger a severe allergic reaction requiring treatment with medications and/or treatment in the ER. Patients with asthma are at increased risk for triggering an asthma attack during testing. You should not undergo testing if you feel that you have allergy or asthma symptoms are currently under poor control. 3. Contraindications to Skin Testing Women who are pregnant or anyone who is currently taking Beta-Blockers, Tricyclic Anti-depressants or MAOI’s medications will NOT be skin tested. Please be sure to inform us of ALL your medications before the skin test is applied. -

Medication Avoidance List for Skin Testing
D. L. Southern, M.D. A. Pedinoff, M.D. J. Caucino, D.O. H. Skolnick, M.D. K. Sikorski, M.D. S. Shah, M.D. N. Baman, M.D. Princeton 609-921-2202 Plainsboro 609-799-8111 Hamilton 609-888-1555 Flemington 908-782-0093 Fax #: 609-924-1468 Your skin testing or food/medication challenge has been scheduled for ______________________________________. The following is a list of commonly used antihistamines and medications, which can interfere with and reduce the accuracy of allergy testing. Please read carefully the times a medication needs to be discontinued prior to testing: Avoid the following antihistamine medications for 3 full days prior to skin testing: Benadryl=diphenydramine Phenergan cough syrup=promethazine Ru-Tuss, Triaminic=pheniramine Polaramine=tripelenamine Dramamine,Bonine=meclizine Bromfed, Dimetapp=brompheniramine Nolahist, Nolamine=phenindamine Actifed=triprolidine Trinalin=azatadine Avoid the following antihistamine medications for 5 full days prior to skin testing: Atarax=hydroxyzine Allegra=fexofenadine Polaramine=dexachlorpheniramine Zyrtec=cetirizine Avoid the following antihistamine medication for 7 full days prior to skin testing: Claritin=loratidine Clarinex=desloratidine Tavist=clemastine Xyzal=levocetirizine Avoid the following antihistamine for 9 full days prior to skin testing: Periactin=cyproheptadine Avoid the following nasal antihistamine sprays for 5 days prior to skin testing: Dymista, Astepro, Astelin=azelastine Patanase=olapatadine Avoid the following stomach medications for 3 days prior to skin testing: Zantac=ranitidine -

Supporting Information a Analysed Substances
Electronic Supplementary Material (ESI) for Analyst. This journal is © The Royal Society of Chemistry 2020 List of contents: Tab. A1 Detailed list and classification of analysed substances. Tab. A2 List of selected MS/MS parameters for the analytes. Tab. A1 Detailed list and classification of analysed substances. drug of therapeutic doping agent analytical standard substance abuse drug (WADA class)* supplier (+\-)-amphetamine ✓ ✓ S6 stimulants LGC (+\-)-methamphetamine ✓ S6 stimulants LGC (+\-)-3,4-methylenedioxymethamphetamine (MDMA) ✓ S6 stimulants LGC methylhexanamine (4-methylhexan-2-amine, DMAA) S6 stimulants Sigma cocaine ✓ ✓ S6 stimulants LGC methylphenidate ✓ ✓ S6 stimulants LGC nikethamide (N,N-diethylnicotinamide) ✓ S6 stimulants Aldrich strychnine S6 stimulants Sigma (-)-Δ9-tetrahydrocannabinol (THC) ✓ ✓ S8 cannabinoids LGC (-)-11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THC-COOH) S8 cannabinoids LGC morphine ✓ ✓ S7 narcotics LGC heroin (diacetylmorphine) ✓ ✓ S7 narcotics LGC hydrocodone ✓ ✓ Cerillant® oxycodone ✓ ✓ S7 narcotics LGC (+\-)-methadone ✓ ✓ S7 narcotics Cerillant® buprenorphine ✓ ✓ S7 narcotics Cerillant® fentanyl ✓ ✓ S7 narcotics LGC ketamine ✓ ✓ LGC phencyclidine (PCP) ✓ S0 non-approved substances LGC lysergic acid diethylamide (LSD) ✓ S0 non-approved substances LGC psilocybin ✓ S0 non-approved substances Cerillant® alprazolam ✓ ✓ LGC clonazepam ✓ ✓ Cerillant® flunitrazepam ✓ ✓ LGC zolpidem ✓ ✓ LGC VETRANAL™ boldenone (Δ1-testosterone / 1-dehydrotestosterone) ✓ S1 anabolic agents (Sigma-Aldrich) -

Wo 2010/075090 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 1 July 2010 (01.07.2010) WO 2010/075090 A2 (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C07D 409/14 (2006.01) A61K 31/7028 (2006.01) kind of national protection available): AE, AG, AL, AM, C07D 409/12 (2006.01) A61P 11/06 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) International Application Number: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US2009/068073 HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 15 December 2009 (15.12.2009) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TJ, TM, TN, TR, TT, (26) Publication Language: English TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/122,478 15 December 2008 (15.12.2008) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, (71) Applicant (for all designated States except US): AUS- ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, PEX PHARMACEUTICALS, INC. -

Ophthalmic Antihistamines
Ophthalmics for Allergic Conjunctivitis Review 04/12/2011 Copyright © 2004 - 2011 by Provider Synergies, L.L.C. All rights reserved. Printed in the United States of America. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage and retrieval system without the express written consent of Provider Synergies, L.L.C. All requests for permission should be mailed to: Attention: Copyright Administrator Intellectual Property Department Provider Synergies, L.L.C. 10101 Alliance Rd, Ste 201 Cincinnati, Ohio 45242 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, -

Epinastine Hcl Ophthalmic Solution) 0.05% Sterile
NDA 21-565/S-005 Page 2 ELESTAT™ (epinastine HCl ophthalmic solution) 0.05% Sterile DESCRIPTION ELESTAT™ (epinastine HCl ophthalmic solution) 0.05% is a clear, colorless, sterile isotonic solution containing epinastine HCl, an antihistamine and an inhibitor of histamine release from the mast cell for topical administration to the eyes. Epinastine HCl is represented by the following structural formula: C16H15N3 • HCl Mol. Wt. 285.78 Chemical Name: 3-Amino-9, 13b-dihydro-1H-dibenz[c,f]imidazo[1,5-a]azepine hydrochloride Each mL contains: Active: Epinastine HCl 0.05% (0.5 mg/mL) equivalent to epinastine 0.044% (0.44mg/mL); Preservative: Benzalkonium chloride 0.01%; Inactives: Edetate disodium; purified water; sodium chloride; sodium phosphate, monobasic; and sodium hydroxide and/or hydrochloric acid (to adjust the pH). ELESTAT™ has a pH of approximately 7 and an osmolality range of 250 to 310 mOsm/kg. CLINICAL PHARMACOLOGY Epinastine is a topically active, direct H1-receptor antagonist and an inhibitor of the release of histamine from the mast cell. Epinastine is selective for the histamine H1-receptor and has affinity for the histamine H2 receptor. Epinastine also possesses affinity for the α1-, α2-, and 5-HT2 –receptors. Epinastine does not penetrate the blood/brain barrier and, therefore, is not expected to induce side effects of the central nervous system. Fourteen subjects, with allergic conjunctivitis, received one drop of ELESTAT™ in each eye twice daily for seven days. On day seven average maximum epinastine plasma concentrations of 0.04 ± 0.014 ng/ml were reached after about two hours indicating low systemic exposure. -

Allergy Skin Testing: Patient Instructions and Consent Form
ALLERGY SKIN TESTING: PATIENT INSTRUCTIONS AND CONSENT FORM Skin tests are a method of testing for allergic reactions to substances, or allergens, in the environment. A test consists of introducing small amounts of allergens into the skin and noting the development of a positive reaction, which consists of a wheal (swelling) and flare (surrounding area of redness). We employ the prick method, where the skin is pricked with a sharp device that introduces the allergen into the skin. Other allergy testing options include injecting the allergen with needles or going to a lab for blood tests. The entire testing process will take about 30 minutes. We test a variety of important allergens that are found in the Central Florida area including trees, grasses, weeds, molds, dust mites, and animal dander. After administering the allergens, we wait approximately 20 minutes to review the results. A positive reaction occurs when the skin becomes red, raised, and itchy. This skin reaction will gradually dissipate within 30‐60 minutes. Some people will experience local swelling beginning 4‐8 hours after testing. This is not serious and typically no treatment is required. It should disappear in the next few days. Less than 1% of patients may develop a systemic reaction to skin testing, which may consist of any or all of the following symptoms: itchy eyes, nose, or throat, nasal congestion, runny nose, tightness in the throat or chest, wheezing, lightheadedness, nausea or vomiting, hives, or anaphylactic shock. This is very rare, but in the event of such reactions, the staff is fully prepared and emergency equipment is readily available. -
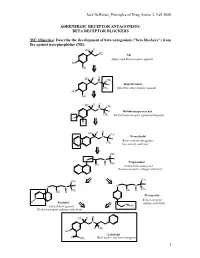
BETA RECEPTOR BLOCKERS MC Objective
Jack DeRuiter, Principles of Drug Action 2, Fall 2000 ADRENERGIC RECEPTOR ANTAGONISTS: BETA RECEPTOR BLOCKERS MC Objective: Describe the development of beta antagonists ("beta blockers") from the agonist norepinephrine (NE): HO H NH2 NE Alpha- and Beta-receptor agonist HO OH H H HO CH N 3 H Isoproterenol CH3 Selective beta-receptor agonist HO OH H H HO CH N 3 H Dichloroisoproterenol CH3 Partial beta-receptor agonist/antagonist Cl Cl H H HO CH N 3 Pronethalol H Beta-receptor antagonist, CH 3 low activity and toxic CH3 O N H Propranolol CH3 OH H Potent beta-antagonist No beta-receptor subtype selectivity CH3 CH3 O N H O N H CH CH OH H 3 OH H 3 Metoprolol N Beta-1-receptor H Pindolol O subtype selectivity Partial beta-agonist CH3 No beta-receptor subtype selectivity HO H H N H CH3 HO Labetolol O NH2 Dual alpha- and beta-antagonst 1 Jack DeRuiter, Principles of Drug Action 2, Fall 2000 MC Objective: Based on their structures, would the beta-blockers be expected to be relatively receptor selective? YES. They do not produce significant blockade of alpha- adrenergic receptors (alpha-1 or alpha-2), histamine receptors, muscarinic receptors or dopamine receptors. MC/PC Objective: Identify which beta blockers are classified as "non-selective": · The “non-selective" classification refers to those beta-blockers capable of blocking BOTH beta-1 and beta-2 receptors with equivalent efficacy. These drugs DO NOT have clinically significant affinity for other neurotransmitter receptors (alpha, dopamine, histamine, acetylcholine, etc.). · ALL of these beta-blockers (except satolol) consist of an aryloxypropanolamine side chain linked to an aromatic or “heteroaromatic” ring which is “ortho” substituted. -

Antihistamines in the Treatment of Chronic Urticaria I Jáuregui,1 M Ferrer,2 J Montoro,3 I Dávila,4 J Bartra,5 a Del Cuvillo,6 J Mullol,7 J Sastre,8 a Valero5
Antihistamines in the treatment of chronic urticaria I Jáuregui,1 M Ferrer,2 J Montoro,3 I Dávila,4 J Bartra,5 A del Cuvillo,6 J Mullol,7 J Sastre,8 A Valero5 1 Service of Allergy, Hospital de Basurto, Bilbao, Spain 2 Department of Allergology, Clínica Universitaria de Navarra, Pamplona, Spain 3 Allergy Unit, Hospital La Plana, Villarreal (Castellón), Spain 4 Service of Immunoallergy, Hospital Clínico, Salamanca, Spain 5 Allergy Unit, Service of Pneumology and Respiratory Allergy, Hospital Clínic (ICT), Barcelona, Spain 6 Clínica Dr. Lobatón, Cádiz, Spain 7 Rhinology Unit, ENT Service (ICEMEQ), Hospital Clínic, Barcelona, Spain 8 Service of Allergy, Fundación Jiménez Díaz, Madrid, Spain ■ Summary Chronic urticaria is highly prevalent in the general population, and while there are multiple treatments for the disorder, the results obtained are not completely satisfactory. The second-generation H1 antihistamines remain the symptomatic treatment option of choice. Depending on the different pharmacokinetics and H1 receptor affi nity of each drug substance, different concentrations in skin can be expected, together with different effi cacy in relation to the histamine-induced wheal inhibition test - though this does not necessarily have repercussions upon clinical response. The antiinfl ammatory properties of the H1 antihistamines could be of relevance in chronic urticaria, though it is not clear to what degree they infl uence the fi nal therapeutic result. Before moving on to another therapeutic level, the advisability of antihistamine dose escalation should be considered, involving increments even above those approved in the Summary of Product Characteristics. Physical urticaria, when manifesting isolatedly, tends to respond well to H1 antihistamines, with the exception of genuine solar urticaria and delayed pressure urticaria. -

CARTEOLOL HYDROCHLORIDE- Carteolol Hydrochloride Solution Sandoz Inc ------Carteolol Hydrochloride Ophthalmic Solution USP, 1% Rx Only Sterile
CARTEOLOL HYDROCHLORIDE- carteolol hydrochloride solution Sandoz Inc ---------- Carteolol Hydrochloride Ophthalmic Solution USP, 1% Rx Only Sterile DESCRIPTION Carteolol Hydrochloride Ophthalmic Solution USP, 1% is a nonselective beta-adrenoceptor blocking agent for ophthalmic use. The chemical name for carteolol hydrochloride is (±)-5-[3-[(1,1-dimethylethyl) amino]-2- hydroxypropoxy]-3,4-dihydro-2(1H)-quinolinone monohydrochloride. The structural formula is as follows: C16H24N2O3•HCI Mol. Wt. 328.84 Each mL of sterile solution contains Active: carteolol hydrochloride 10 mg (1%). Preservative: benzalkonium chloride 0.05 mg (0.005%). Inactives: sodium chloride, monobasic and dibasic sodium phosphate, sodium hydroxide and/or hydrochloric acid (to adjust pH to 6.0 - 8.0) and purified water. CLINICAL PHARMACOLOGY Carteolol is a nonselective beta-adrenergic blocking agent with associated intrinsic sympathomimetic activity and without significant membrane-stabilizing activity. Carteolol Hydrochloride reduces normal and elevated intraocular pressure (IOP) whether or not accompanied by glaucoma. The exact mechanism of the ocular hypotensive effect of beta-blockers has not been definitely demonstrated. In general, beta-adrenergic blockers reduce cardiac output in patients in good and poor cardiovascular health. In patients with severe impairment of myocardial function, beta-blockers may inhibit the sympathetic stimulation necessary to maintain adequate cardiac function. Beta-adrenergic blockers may also increase airway resistance in the bronchi and bronchioles due to unopposed parasympathetic activity. Given topically twice daily in controlled domestic clinical trials ranging from 1.5 to 3 months, Carteolol Hydrochloride produced a median percent reduction of IOP 22% to 25%. No significant effects were noted on corneal sensitivity, tear secretion, or pupil size. -

Pharmaceuticals As Environmental Contaminants
PharmaceuticalsPharmaceuticals asas EnvironmentalEnvironmental Contaminants:Contaminants: anan OverviewOverview ofof thethe ScienceScience Christian G. Daughton, Ph.D. Chief, Environmental Chemistry Branch Environmental Sciences Division National Exposure Research Laboratory Office of Research and Development Environmental Protection Agency Las Vegas, Nevada 89119 [email protected] Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Why and how do drugs contaminate the environment? What might it all mean? How do we prevent it? Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada This talk presents only a cursory overview of some of the many science issues surrounding the topic of pharmaceuticals as environmental contaminants Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada A Clarification We sometimes loosely (but incorrectly) refer to drugs, medicines, medications, or pharmaceuticals as being the substances that contaminant the environment. The actual environmental contaminants, however, are the active pharmaceutical ingredients – APIs. These terms are all often used interchangeably Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Office of Research and Development Available: http://www.epa.gov/nerlesd1/chemistry/pharma/image/drawing.pdfNational -

Proposed Changes to the 2021 FEI Equine Prohibited Substances List (EPSL) (Effective from 01.01.2022)
Proposed changes to the 2021 FEI Equine Prohibited Substances List (EPSL) (effective from 01.01.2022) SUBSTANCE NAME CURRENT PROPOSED COMMENT STATUS EPSL CATEGORY ACETYLCHOLINESTERASE INHIBITOR Used in the treatment of Edrophonium Controlled Banned myasthenia gravis and Medication Substance has no legitimate use in the treatment of sports horses Used to increase Huperzine A Unlisted Banned alertness and treat Substance myasthenia gravis. The substance has no legitimate use in the treatment of sports horses. AMIDES Palmitoylethanolamid Banned Controlled Used in the treatment of Substance Medication joint pain ANGIOTENSIN CONVERTING ENZYME INHIBITORS Enalapril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Enalaprilat Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Lisinopril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Moexipril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse 1 Perindoprilat Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse ANTIHISTAMINES Antazoline Controlled Banned The substance has no Medication Substance legitimate use in the sports horse Azatadine Controlled Banned The substance has Medication Substance sedative effects