Medications Which Must Be Withheld Prior to Skin Testing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MSM Cross Reference Antihistamine Decongestant 20100701 Final Posted
MISSISSIPPI DIVISION OF MEDICAID Antihistamine/Decongestant Product and Active Ingredient Cross-Reference List The agents listed below are the antihistamine/decongestant drug products listed in the Mississippi Medicaid Preferred Drug List (PDL). This is a cross-reference between the drug product name and its active ingredients to reference the antihistamine/decongestant portion of the PDL. For more information concerning the PDL, including non- preferred agents, the OTC formulary, and other specifics, please visit our website at www.medicaid.ms.gov. List Effective 07/16/10 Therapeutic Class Active Ingredients Preferred Non-Preferred ANTIHISTAMINES - 1ST GENERATION BROMPHENIRAMINE MALEATE BPM BROMAX BROMPHENIRAMINE MALEATE J-TAN PD BROMSPIRO LODRANE 24 LOHIST 12HR VAZOL BROMPHENIRAMINE TANNATE BROMPHENIRAMINE TANNATE J-TAN P-TEX BROMPHENIRAMINE/DIPHENHYDRAM ALA-HIST CARBINOXAMINE MALEATE CARBINOXAMINE MALEATE PALGIC CHLORPHENIRAMINE MALEATE CHLORPHENIRAMINE MALEATE CPM 12 CHLORPHENIRAMINE TANNATE ED CHLORPED ED-CHLOR-TAN MYCI CHLOR-TAN MYCI CHLORPED PEDIAPHYL TANAHIST-PD CLEMASTINE FUMARATE CLEMASTINE FUMARATE CYPROHEPTADINE HCL CYPROHEPTADINE HCL DEXCHLORPHENIRAMINE MALEATE DEXCHLORPHENIRAMINE MALEATE DIPHENHYDRAMINE HCL ALLERGY MEDICINE ALLERGY RELIEF BANOPHEN BENADRYL BENADRYL ALLERGY CHILDREN'S ALLERGY CHILDREN'S COLD & ALLERGY COMPLETE ALLERGY DIPHEDRYL DIPHENDRYL DIPHENHIST DIPHENHYDRAMINE HCL DYTUSS GENAHIST HYDRAMINE MEDI-PHEDRYL PHARBEDRYL Q-DRYL QUENALIN SILADRYL SILPHEN DIPHENHYDRAMINE TANNATE DIPHENMAX DOXYLAMINE SUCCINATE -

2-Bromopyridine Safety Data Sheet Jubilant Ingrevia Limited
2-Bromopyridine Safety Data Sheet According to the federal final rule of hazard communication revised on 2012 (HazCom 2012) Date of Compilation : July 03 ’ 2019 Date of Revision : February 09 ’ 2021 Revision due date : January 2024 Revision Number : 01 Version Name : 0034Gj Ghs01 Div.3 sds 2-Bromopyridine Supersedes date : July 03 ’ 2019 Supersedes version : 0034Gj Ghs00 Div.3 sds 2-Bromopyridine Jubilant Ingrevia Limited Page 1 of 9 2-Bromopyridine Safety Data Sheet According to the federal final rule of hazard communication revised on 2012 (HazCom 2012) SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier PRODUCT NAME : 2-Bromopyridine CAS RN : 109-04-6 EC# : 203-641-6 SYNONYMS : 2-Pyridyl bromide, Pyridine, 2-bromo-, beta-Bromopyridine, o-Bromopyridine SYSTEMATIC NAME : 2-Bromopyridine, -Pyridine, 2-bromo- MOLECULAR FORMULA : C5H4BrN STRUCTURAL FORMULA N Br 1.2. Relevant identified uses of the substance or mixture and uses advised against 1.2.1. Relevant identified uses 2-Bromopyridine is used as an intermediate in the pharmaceutical industry for the manufacture of Atazanavir (an antiretroviral drug), Carbinoxamine, Chloropyramine, triprolidine (antihistamine drugs), Disopyramide Phosphate (an antiarrythmic drug), Mefloquine (antimalarial drug), Pipradrol (mild CNS stimulant) etc. Uses advised against: None 1.3. Details of the supplier of the safety data sheet Jubilant Ingrevia Limited REGISTERED & FACTORY OFFICE: Jubilant Ingrevia Limited Bhartiagram, Gajraula , District: Amroha, Uttar Pradesh-244223, India PHONE NO: +91-5924-252353 to 252360 Contact Department-Safety: Ext. 7424 , FAX NO : +91-5924-252352 HEAD OFFICE: Jubilant Ingrevia Limited, Plot 1-A, Sector 16-A,Institutional Area, Noida, Uttar Pradesh, 201301 - India T +91-120-4361000 - F +91-120-4234881 / 84 / 85 / 87 / 95 / 96 [email protected] -www.jubilantingrevia.com 1.4. -

Medication Avoidance List for Skin Testing
D. L. Southern, M.D. A. Pedinoff, M.D. J. Caucino, D.O. H. Skolnick, M.D. K. Sikorski, M.D. S. Shah, M.D. N. Baman, M.D. Princeton 609-921-2202 Plainsboro 609-799-8111 Hamilton 609-888-1555 Flemington 908-782-0093 Fax #: 609-924-1468 Your skin testing or food/medication challenge has been scheduled for ______________________________________. The following is a list of commonly used antihistamines and medications, which can interfere with and reduce the accuracy of allergy testing. Please read carefully the times a medication needs to be discontinued prior to testing: Avoid the following antihistamine medications for 3 full days prior to skin testing: Benadryl=diphenydramine Phenergan cough syrup=promethazine Ru-Tuss, Triaminic=pheniramine Polaramine=tripelenamine Dramamine,Bonine=meclizine Bromfed, Dimetapp=brompheniramine Nolahist, Nolamine=phenindamine Actifed=triprolidine Trinalin=azatadine Avoid the following antihistamine medications for 5 full days prior to skin testing: Atarax=hydroxyzine Allegra=fexofenadine Polaramine=dexachlorpheniramine Zyrtec=cetirizine Avoid the following antihistamine medication for 7 full days prior to skin testing: Claritin=loratidine Clarinex=desloratidine Tavist=clemastine Xyzal=levocetirizine Avoid the following antihistamine for 9 full days prior to skin testing: Periactin=cyproheptadine Avoid the following nasal antihistamine sprays for 5 days prior to skin testing: Dymista, Astepro, Astelin=azelastine Patanase=olapatadine Avoid the following stomach medications for 3 days prior to skin testing: Zantac=ranitidine -

Medication Instructions for Allergy Patients
MEDICATION INSTRUCTIONS FOR ALLERGY PATIENTS Drugs which contain antihistamine or have antihistaminic effects can result in negative reactions to skin testing. As a result, it may not be possible to properly interpret skin test results, and testing may have to be repeated at a later date. While this list is extensive, it is NOT all inclusive (particularly of the various brand names). Discontinue ALL antihistamines including the following medications seven (7) days prior to skin testing (unless longer time specified): Antihistamines – Generic name (Brand name(s)): Cetirizine (Zyrtec, Zyrtec-D) Hydroxyzine (Vistaril, Atarax) Desloratadine (Clarinex) Levocetirizine (Xyzal) Fexofenadine (Allegra, Allegra-D) Loratadine (Claritin, Claritin-D, Alavert) Diphenhydramine (Aleve PM, Benadryl, Bayer P.M., Benylin, Contac P.M., Doans P.M, Excedrin PM, Legatrin P.M.. Nytol, Tylenol Nighttime, Unisom, Zzzquil) Chlorpheniramine (Aller-Chlor, Allerest, Alka Seltzer Plus, Chlor-Trimeton, Comtrex, Contac, Co-Pyronil, Coricidin, CTM, Deconamine, Dristan, Dura-tap, Naldecon, Ornade Spansules, Rondec, Sinutab, Teldrin, Triaminic, Triaminicin, Tylenol Allergy) Azatadine (Optimine, Trinalin) Doxylamine (Nyquil) Brompheniramine (Bromfed, Dimetane, Dimetapp) Meclizine (Antivert) Carbinoxamine (Clistin, Rondec) Pheniramine Clemastine (Tavist) Phenyltoloxamine (Nadecon) Cyclizine (Marezine) Promethazine (Phenergan) Cyprohepatidine (Periactin) (9 days) Pyrilamine (Mepyramine) Dexbrompheniramine (Drixoral) Quinacrine (Atabrine) Dexchlorpheniramine (Extendryl, Polaramine) -

Antihistamine Therapy in Allergic Rhinitis
CLINICAL REVIEW Antihistamine Therapy in Allergic Rhinitis Paul R. Tarnasky, MD, and Paul P. Van Arsdel, Jr, MD Seattle, Washington Allergic rhinitis is a common disorder that is associated with a high incidence of mor bidity and considerable costs. The symptoms of allergic rhinitis are primarily depen dent upon the tissue effects of histamine. Antihistamines are the mainstay of therapy for allergic rhinitis. Recently, a second generation of antihistamines has become available. These agents lack the adverse effect of sedation, which is commonly associated with older antihistamines. Current practice of antihistamine therapy in allergic rhinitis often involves random selection among the various agents. Based upon the available clinical trials, chlorpheniramine appears to be the most reasonable initial antihistaminic agent. A nonsedating antihis tamine should be used initially if a patient is involved in activities where drowsiness is dangerous. In this comprehensive review of allergic rhinitis and its treatment, the cur rent as well as future options in antihistamine pharmacotherapy are emphasized. J Fam Pract 1990; 30:71-80. llergic rhinitis is a common condition afflicting some defined by the period of exposure to those agents to which A where between 15 and 30 million people in the United a patient is sensitive. Allergens in seasonal allergic rhinitis States.1-3 The prevalence of disease among adolescents is consist of pollens from nonflowering plants such as trees, estimated to be 20% to 30%. Two thirds of the adult grasses, and weeds. These pollens generally create symp allergic rhinitis patients are under 30 years of age.4-6 Con toms in early spring, late spring through early summer, sequently, considerable costs are incurred in days lost and fall, respectively. -

Trends in Prescription Medication Use Among Children and Adolescents— United States, 1999-2014
Supplementary Online Content Hales CM, Kit BK, Gu Q, Ogden CL. Trends in prescription medication use among children and adolescents— United States, 1999-2014. JAMA. doi:10.1001/jama.2018.5690 eTable. Classification of Prescription Medications Reported by NHANES Participants Aged 0-19 Years From 1999- 2000 to 2013-2014 by Therapeutic Class This supplementary material was provided by the authors to give readers additional informtion about their work. © 2018 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 09/27/2021 eTable. Classification of Prescription Medications Reported by NHANES Participants Aged 0-19 Years From 1999- 2000 to 2013-2014 by Therapeutic Class Therapeutic classes are based on the Lexicon Plus prescription medication database and only those classes reported in the manuscript are listed. ADHD Medications Antiadrenergic Agents, Centrally Acting Clonidine Guanfacine CNS Stimulants Amphetamines Amphetamine Amphetamine; Dextroamphetamine Dextroamphetamine Lisdexamfetamine Methylphenidate or Dexmethylphenidate Dexmethylphenidate Methylphenidate Other CNS Stimulant Pemoline Selective Norepinephrine Reuptake Inhibitor Atomoxetine Antibiotics Cephalosporins Cefadroxil Cephalexin Cefaclor Cefprozil Cefuroxime Loracarbef Cefdinir Cefditoren Cefixime Cefpodoxime Ceftibuten Ceftriaxone Glycopeptide Antibiotics Vancomycin H. Pylori Eradication Agents Amoxicillin; Clarithromycin; Lansoprazole Lincomycin Derivatives Clindamycin Macrolide Derivatives Telithromycin Azithromycin Clarithromycin -

Medication Guide for a Safe Recovery
Medication Guide For A Safe Recovery A guide to maintaining sobriety while receiving treatment for other health problems. Revision 1.0 -April 2008 Table of Contents Introduction..................................................................................2 How to Use this Guide..................................................................3 Class A Drugs (Absolutely Avoid)................................................4 Class B Drugs................................................................................8 (With Addiction Medicine Specialist/Doctor Approval Only) Class C Drugs (Generally Safe to Take).....................................12 Alcohol-Free Products..................................................................16 Incidental Exposure Index...........................................................22 www.talbottcampus.com Introduction From the Talbott Recovery Campus Welcome to the Talbott Recovery Campus guide for a safe and sustained recovery. This document was developed through a collaborative effort between some of the best minds in addiction care today and will help you make wise decisions, ensuring that medications you may be prescribed and incidental exposure to alcohol do not threaten your hard won recovery. This guide is divided into three sections and is based on the drug classification system developed nearly 20 years ago by Dr. Paul Earley and recently expanded on by Bruce Merkin, M.D., Renee Enstrom, Nicholas Link and the staff at Glenbeigh hospital. Part one provides a way of categorizing medications -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2006) – Supplement 1 (Rev. 1) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2006) – Supplement 1 (Rev. 1) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABACAVIR 136470-78-5 ACEXAMIC ACID 57-08-9 ABAFUNGIN 129639-79-8 ACICLOVIR 59277-89-3 ABAMECTIN 65195-55-3 ACIFRAN 72420-38-3 ABANOQUIL 90402-40-7 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABCIXIMAB 143653-53-6 ACITEMATE 101197-99-3 ABECARNIL 111841-85-1 ACITRETIN 55079-83-9 ABIRATERONE 154229-19-3 ACIVICIN 42228-92-2 ABITESARTAN 137882-98-5 ACLANTATE 39633-62-0 ABLUKAST 96566-25-5 ACLARUBICIN 57576-44-0 ABUNIDAZOLE 91017-58-2 ACLATONIUM NAPADISILATE 55077-30-0 ACADESINE 2627-69-2 ACODAZOLE 79152-85-5 ACAMPROSATE 77337-76-9 ACONIAZIDE 13410-86-1 ACAPRAZINE 55485-20-6 ACOXATRINE 748-44-7 ACARBOSE 56180-94-0 ACREOZAST 123548-56-1 ACEBROCHOL 514-50-1 ACRIDOREX 47487-22-9 ACEBURIC -

San Diego ENT
San Diego ENT Allergy Skin Testing Instructions Make sure you review all of your medications with your doctor or the medical assistant when you are scheduled for your allergy test. DON’T’S: • Do note take over-the-counter antihistamines, cold medication, or cough syrup for 10 days prior to the test. This includes Benadryl, Claritin, Zyrtec, Allegra, loratadine, cetirizine, and fexofenadine, Tavist, Dramamine,Atarax, and others. • Do not take prescription antihistamines for 10 days prior to the test including Claritin, Zyrtec, Allegra, loratadine, cetirizine, fexofenadine, and Astelin nasal spray. Also stop antihistamine eye drops 10 days prior to testing. • Do not take beta blockers 5 days prior to testing. These include labetalol, metoprolol, carvedilol, and their brand name equivalents. • Do not take anti-acid medication for 48 hours prior to testing including Zantac, Pepcid, and Tagamet. You may continue to take proton pump inhibitors such as Prilosec, Nexium, Prevacid, Protonix, and Aciphex. • Do not take any sleeping medications for 48 hours prior to testing including Tylenol PM and Excedrin PM. DO’S: • Wear something comfortable that will allow access to your back or both upper arms on the day of testing. • You may continue to use nasal steroid sprays such as Flonase, Nasonex, Nasacort, and Rhinocort. Do not use Astelin. • Review the list of medications that need to be avoided below. Antihistamines to stop 10 days prior to testing: Generic Brand Name Acrivastine Semprex Azatadine Optimine, Trinalin Bropheniramine AccuHist, Bromfed, -
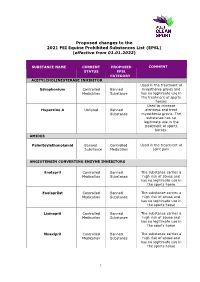
Proposed Changes to the 2021 FEI Equine Prohibited Substances List (EPSL) (Effective from 01.01.2022)
Proposed changes to the 2021 FEI Equine Prohibited Substances List (EPSL) (effective from 01.01.2022) SUBSTANCE NAME CURRENT PROPOSED COMMENT STATUS EPSL CATEGORY ACETYLCHOLINESTERASE INHIBITOR Used in the treatment of Edrophonium Controlled Banned myasthenia gravis and Medication Substance has no legitimate use in the treatment of sports horses Used to increase Huperzine A Unlisted Banned alertness and treat Substance myasthenia gravis. The substance has no legitimate use in the treatment of sports horses. AMIDES Palmitoylethanolamid Banned Controlled Used in the treatment of Substance Medication joint pain ANGIOTENSIN CONVERTING ENZYME INHIBITORS Enalapril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Enalaprilat Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Lisinopril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Moexipril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse 1 Perindoprilat Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse ANTIHISTAMINES Antazoline Controlled Banned The substance has no Medication Substance legitimate use in the sports horse Azatadine Controlled Banned The substance has Medication Substance sedative effects -
![With [3H]Mepyramine (Trieyclic Antidepressants/Antihistamine/Neurotransmitter/Amitriptyline) VINH TAN TRAN, RAYMOND S](https://docslib.b-cdn.net/cover/2862/with-3h-mepyramine-trieyclic-antidepressants-antihistamine-neurotransmitter-amitriptyline-vinh-tan-tran-raymond-s-1512862.webp)
With [3H]Mepyramine (Trieyclic Antidepressants/Antihistamine/Neurotransmitter/Amitriptyline) VINH TAN TRAN, RAYMOND S
Proc. Nati. Acad. Sci. USA Vol. 75, No. 12, pp. 6290-6294,, December 1978 Neurobiology Histamine H1 receptors identified in mammalian brain membranes with [3H]mepyramine (trieyclic antidepressants/antihistamine/neurotransmitter/amitriptyline) VINH TAN TRAN, RAYMOND S. L. CHANG, AND SOLOMON H. SNYDER* Departments of Pharmacology and Experimental Therapeutics, and Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205 Communicated by Julius Axelrod, August 30,1978 ABSTRACT The antihistamine [3H mepyramine binds to Male Sprague-Dawley rats (150-200 g) were killed by cer- HI histamine receptors in mammalian brain membranes. vical dislocation, their brains were rapidly removed and ho- Potencies of H1 antihistamines at the binding sites correlate mogenized with a Polytron for 30 min (setting 5) in 30 vol of with their pharmacological antihistamine effects in the guinea pig ileum. Specific [3Himepyramine binding is saturable with ice-cold Na/K phosphate buffer (50 mM, pH 7.5), and the a dissociation constant of about 4 nM in both equilibrium and suspension was centrifuged (50,000 X g for 10 min). The pellet kinetic experiments and a density of 10pmolper gram ofwhole was resuspended in the same volume of fresh buffer and cen- brain. Some tricyclic antidepressants are potent inhibitors of trifuged, and the final pellet was resuspended in the original secific [3Hmepamine binding. Regional variations of volume of ice-cold buffer by Polytron homogenization. Calf [3Hjmepyramine ing do not correlate with variations in brains were obtained from a local abattoir within 2 hr after the endogeneous histamine and histidine decarboxylase activity. death of the animals and transferred to the laboratory in ice- Histamine is a neurotransmitter candidate in mammalian brain cold saline. -

Ryan White Part a Formulary
Ryan White Part A Formulary Updated ƵŐƵƐƚ 2019 Ryan White Part A Pharmacy Formulary - Updated 8/19/19 Tier 1: In order to access Tier 1, clients must qualify for the Ryan White Part A Program based on eligiblity requirements. Drug Classification Brand Name Generic Name Tier 5-Alpha-Reductase Inhibitors/BPH Avodart Dutasteride 1 Anticoagulant/Factor Xa Inhibitors Xarelto Rivaroxaban 1 Antihyperlipidemic Agents- Dietary Source Omega 3 Alpha-Linoleic Acid 1 Antiinfectives/Antifungal Lamisil Terbinafine 1 Antiinfectives/Antiinflammatory Decadron Dexamethasone 1 Antiinfectives/Antiinflammatory Deltasone Prednisone 1 Antiinfectives/Antiinflammatory Medrol dosepak Methyprednisolone 1 Antiinfectives/Antitubercular Rifamate Isoniazid/Rifampin 1 Antiinfectives/Antitubercular Seromycin Cycloserine 1 Antiinfectives/Antiviral/Antibacterial Achromycin Tetracycline 1 Antiinfectives/Antiviral/Antibacterial Amoxil Amoxicillin 1 Antiinfectives/Antiviral/Antibacterial Augmentin Amoxicillin/Clavulanate Potassium 1 Antiinfectives/Antiviral/Antibacterial Bicillin LA Penicillin Benzath 1 Antiinfectives/Antiviral/Antibacterial Ceftin Cefuroxime 1 Antiinfectives/Antiviral/Antibacterial Cipro Ciprofloxacin 1 Antiinfectives/Antiviral/Antibacterial Cleocin Clindamycin 1 Antiinfectives/Antiviral/Antibacterial Ery-tab Erythromycin base 1 Antiinfectives/Antiviral/Antibacterial Keflex Cephalexin 1 Antiinfectives/Antiviral/Antibacterial Macrodantin Nitrofurantoin 1 Antiinfectives/Antiviral/Antibacterial Omnicef Cefdinir 1 Antiinfectives/Antiviral/Antibacterial PenVK Penicililn