Rediscovery of a Presumed Extinct Species, Salvelinus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluating 87Sr/86Sr Isotope Ratios and Sr Mass Fractions in Otoliths Of
bioRxiv preprint doi: https://doi.org/10.1101/2021.07.23.453494; this version posted July 25, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Evaluating 87Sr/86Sr isotope ratios and Sr mass fractions in otoliths of different European freshwater 2 fish species as fishery management tool in an Alpine foreland with limited geological variability 3 Andreas Zitek1,2*, Johannes Oehm3, Michael Schober1, Anastassiya Tchaikovsky1, Johanna Irrgeher5, 4 Anika Retzmann5, Bettina Thalinger4, Michael Traugott3, Thomas Prohaska5 5 1University of Natural Resources and Life Sciences, Vienna, Department of Chemistry, Institute of 6 Analytical Chemistry, Muthgasse 18, 1190 Wien, Austria 7 2FFoQSI GmbH ‐ Austrian Competence Centre for Feed and Food Quality, Safety & Innovation, 8 Technopark 1D, 3430 Tulln, Austria 9 3University of Innsbruck, Department of Zoology, Technikerstraße 25, 6020 Innsbruck, Austria 10 4University of Guelph, 50 Stone Road East, Guelph, N1G2W1, Canada 11 5Department of General, Analytical and Physical Chemistry, Chair of General and Analytical Chemistry, 12 Montanuniversität Leoben, Franz Josef‐Straße 18, 8700 Leoben, Austria 13 14 *Corresponding author: [email protected] 15 16 Highlights 17 Otolith microchemistry applied in in area with limited geological variability 18 Fish transferred, stocked or migrated were identified 19 Regressions between Sr/Ca ratios in water predict Sr mass fractions in otoliths 20 Species specific Sr discrimination from water into otoliths 21 European freshwater fish species assigned to habitat clusters of origin 22 Keywords 23 Strontium isotopes, Sr elemental fingerprint, otolith microchemistry, freshwater fish species, fishery 24 management. -
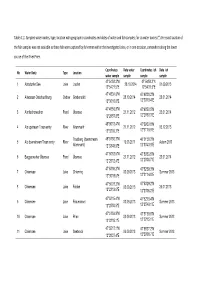
Sampled Water Bodies, Type, Location with Geographic
Table A.1: Sampled water bodies, type, location with geographic coordinates and dates of water and fish samples; for six water bodies (*) the exact location of the fish samples was not available as these fish were captured by fishermen within the investigated lakes, or in one occasion, somewhere along the lower course of the River Prien. Coordinates Date water Coordinates fish Date fish No Water Body Type Location water sample sample sample sample 47°54'52.3"N 47°54'52.3"N 1 Abtsdorfer See Lake Laufen 28.10.2014 01.09.2013 12°54'21.5"E 12°54'21.5"E 47°48'34.6"N 47°48'30.2"N 2 Altwasser Osterbuchberg Oxbow Grabenstätt 28.10.2014 23.01.2014 12°30'10.6"E 12°30'19.4"E 47°48'56.8"N 47°48'53.5"N 3 Almfischerweiher Pond Übersee 21.11.2012 23.01.2014 12°29'57.8"E 12°29'53.3"E 48°00'13.4"N 47°59'53.8"N 4 Alz upstream Traun entry River Altenmarkt 21.11.2012 05.12.2013 12°32'00.3"E 12°31'19.9"E Trostberg (downstream 48°01'50.2"N 48°01'50.2"N 5 Alz downstream Traun entry River 10.03.2011 Autum 2013 Altenmarkt) 12°33'43.9"E 12°33'43.9"E 47°50'52.5"N 47°50'53.5"N 6 Baggerweiher Übersee Pond Übersee 21.11.2012 23.01.2014 12°29'12.4"E 12°29'08.7"E 47°53'09.2"N 47°52'59.3"N 7 Chiemsee Lake Chieming 03.09.2013 Summer 2013 12°30'19.5"E 12°31'14.8"E 47°50'28.3"N 47°50'29.2"N 8 Chiemsee Lake Felden 03.09.2013 26.07.2013 12°23'12.6"E 12°23'06.2"E 47°52'15.4"N 47°52'10.4"N 9 Chiemsee Lake Fraueninsel 03.09.2013 Summer 2013 12°25'50.5"E 12°25'49.1"E 47°51'59.8"N 47°51'59.8"N 10 Chiemsee Lake Prien 03.09.2013 Summer 2013 12°22'15.1"E 12°22'15.1"E 47°55'17.1"N -

09-761 Eindrapport Forellen
A risk analysis of exotic trout in the Netherlands D. M. Soes P.-B. Broeckx Consultants for environment & ecology A risk analysis of exotic trout in the Netherlands D.M. Soes P.-B. Broeckx Commissioned by: Food and Consumer Product Safety Authority 9th of September 2010 Report nr 10-144 Status: Final report Report nr.: 10-144 Date of publication: 9th of September 2010 Title: A risk analysis of exotic trout in the Netherlands Author: Ir. D.M. Soes Ir. P.-B. Broeckx Number of pages without appendices: 96 Project nr: 09-761 Project manager: Ir. D.M. Soes Name & address client: Food and Consumer Product Safety Authority, Invasive Alien Species Team, P.O. Box 9102, 6700 HC, Wageningen Reference client: TRCPD/2009/3834 Signed for publication: General director Bureau Waardenburg bv drs. J.L. Spier Initials: Bureau Waardenburg bv is not liable for any resulting damage, nor for damage which results from applying results of work or other data obtained from Bureau Waardenburg bv; client indemnifies Bureau Waardenburg bv against third-party liability in relation to these applications. © Bureau Waardenburg bv / Food and Consumer Product Safety Authority This report is produced at the request of the client mentioned above and is his property. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, transmitted and/or publicized in any form or by any means, electronic, electrical, chemical, mechanical, optical, photocopying, recording or otherwise, without prior written permission of the client mentioned above and Bureau Waardenburg bv, nor may it without such a permission be used for any other purpose than for which it has been produced. -

Rediscovery of a Presumed Extinct
The Scientific Naturalist Ecology, 0(0), 2020, e03065 © 2020 by the Ecological Society of America they were therefore declared extinct according to IUCN criteria (Freyhof and Kottelat 2008a, b). Today, C. gut- turosus is considered a prime example for speciation reversal, whereby a change in environmental conditions, which are necessary to maintain reproductive isolation Rediscovery of a presumed extinct among evolutionarily young species, causes species to go species, Salvelinus profundus, after extinct by collapsing into a hybrid swarm (Vonlanthen et al. 2012; D. Frei, unpublished data). The simultaneous re-oligotrophication disappearance of the ecologically similar S. profundus led to the conclusion that it shared the fate of C. guttur- 1,2,3 1,2 CARMELA J. D OENZ AND OLE SEEHAUSEN osus (Vonlanthen et al. 2012). Manuscript received 6 December 2019; revised 29 January However, in 2012, a colleague, Jasminca Behrmann- 2020; accepted 25 February 2020. Corresponding Editor: John Godel, informed us about a small, pale, summer- Pastor. spawning charr that she and others had caught. On 1 Department of Fish Ecology & Evolution, Centre for several previous occasions, small individuals of S. um- Ecology, Evolution and Biogeochemistry, EAWAG, bla had wrongly been identified as S. profundus (Kotte- Kastanienbaum, 6047 Switzerland. lat and Freyhof 2007), so in this case, we were hopeful 2Department of Aquatic Ecology & Evolution, Institute of that it could be the presumably extinct species, since it Ecology and Evolution, University of Bern, Bern, 3012 Switzerland. showed several of its traits. In 2010, a large project 3E-mail: [email protected] (Projet Lac) started to assess the fish community for each of the large pre-alpine lakes in and around Citation: Doenz, C. -

Identification and Modelling of a Representative Vulnerable Fish Species for Pesticide Risk Assessment in Europe
Identification and Modelling of a Representative Vulnerable Fish Species for Pesticide Risk Assessment in Europe Von der Fakultät für Mathematik, Informatik und Naturwissenschaften der RWTH Aachen University zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigte Dissertation vorgelegt von Lara Ibrahim, M.Sc. aus Mazeraat Assaf, Libanon Berichter: Universitätsprofessor Dr. Andreas Schäffer Prof. Dr. Christoph Schäfers Tag der mündlichen Prüfung: 30. Juli 2015 Diese Dissertation ist auf den Internetseiten der Universitätsbibliothek online verfügbar Erklärung Ich versichere, dass ich diese Doktorarbeit selbständig und nur unter Verwendung der angegebenen Hilfsmittel angefertigt habe. Weiterhin versichere ich, die aus benutzten Quellen wörtlich oder inhaltlich entnommenen Stellen als solche kenntlich gemacht zu haben. Lara Ibrahim Aachen, am 18 März 2015 Zusammenfassung Die Zulassung von Pflanzenschutzmitteln in der Europäischen Gemeinschaft verlangt unter anderem eine Abschätzung des Risikos für Organismen in der Umwelt, die nicht Ziel der Anwendung sind. Unvertretbare Auswirkungen auf den Naturhalt sollen vermieden werden. Die ökologische Risikoanalyse stellt die dafür benötigten Informationen durch eine Abschätzung der Exposition der Organismen und der sich daraus ergebenden Effekte bereit. Die Effektabschätzung beruht dabei hauptsächlich auf standardisierten ökotoxikologischen Tests im Labor mit wenigen, oft nicht einheimischen Stellvertreterarten. In diesen Tests werden z. B. Effekte auf das Überleben, das Wachstum und/oder die Reproduktion von Fischen bei verschiedenen Konzentrationen der Testsubstanz gemessen und Endpunkte wie die LC50 (Lethal Concentrations for 50%) oder eine NOEC (No Observed Effect Concentration, z. B. für Wachstum oder Reproduktionsparameter) abgeleitet. Für Fische und Wirbeltiere im Allgemeinen beziehen sich die spezifischen Schutzziele auf das Überleben von Individuen und die Abundanz und Biomasse von Populationen. -

Managing Upper Lake Constance Fishery in a Multi- Sector Policy Landscape: Beneficiary and Victim of a Century of Anthropogenic Trophic Change
Managing Upper Lake Constance Fishery in a Multi- Sector Policy Landscape: Beneficiary and Victim of a Century of Anthropogenic Trophic Change Jan Baer1, Reiner Eckmann2, Roland Rösch1, Robert Arlinghaus3 & Alexander Brinker1 1Fisheries Research Station, Langenargen, Germany, [email protected] 2University of Konstanz, Germany 3Faculty of Life Sciences, Humboldt-Universität zu Berlin & Department of Biology and Ecology of Fishes, Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Germany Abstract Upper Lake Constance (ULC) is a large pre-alpine lake situated between Austria, Germany and Switzerland (9°18'E, 47°39'N). Along with the smaller, conjoined expanse of Lower Lake Constance, it forms the third largest lake in Europe. Its waters underwent pronounced eutrophication during the 20th century. Commercial fisheries benefitted strongly from the increased productivity during an initial mesotrophic phase, but these advantages were effectively neutralized when eutrophication became severe. By the turn of the 21st century, internationally coordinated measures to reduce nutrient input to the lake had returned ULC to its historic reference state as an oligotrophic ecosystem. However, the remarkable success of the nutrient management program has been to the detriment of commercial fishers. Yields of most commercially important fish species have decreased, along with lake productivity. As a consequence, the high market demand for local fish products is nowadays met mainly by imports, the ecological footprint of which offsets the local benefits of environmental restoration. Responsibility for fisheries and environmental aspects of ULC managing is shared by the national and federal state administrations and in all cases, tourism, drinking water and environmental interests now take priority over fisheries. -

An Annotated Bibliography of Interspecific Hybridization
FAO Fisheries Circular No.133 FIRI/C133 (Distribution restricted) AN ANNOTATED BIBLIOGRAPHY OF INTERSPECIFIC HYBRIDIZATION OF SALMONIDAE Compiled by James R. Dangel College of Fisheries University of Washington FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, September 1973 PREPARATION OF THIS DOCUMENT This bibliography is an attempt by the author to include all known literature, pub- lished and unpublished, on hybridization in Salmonidae. The whitefishes and graylings are considered by the author as separate families and are not included. The author would appreciate being informed of any references to salmonoid hybrids known to the reader that are not included in this bibliography, as well as corrections or additions to the annotations, so that tuey may be included in future revisions or addenda. Articles not obtained for review are included but not annotated unless referred to in other sources. When abstracts or summaries pertaining to hybridization were included in papers, they have been transcribed in quotation marks verbatim, as are certain passages of text when applicable. Unless otherwise cited, the abstracts were written by the author. WI/E2219 FAO Fisheries Circular (FAO Fish.Circ.) A vehicle for distribution of short or ephemeral notes, lists, etc., including provisional versions of documents to be issued later in other series. 1 Ackermann, K. (1898) 001 Alm, G. (1959) 006 Abh.Ber.Ver.Naturkd.Kassel, 43:4-11 Rep.Inst.Freshwat.Res.Drottningholm, Thierbastarde. Zusammenstellung der T40):5-145 bisherigen Beobachtungen Uber Bastardirung Connection between maturity, size and im Thierreiche nebst Litteraturnachweisen. age in fishes 2. Theil: Die Wirbelthiere (Fische) Salmo salar and S. -

Preliminary Study of the Habitat, Hydrological And
LIFE project number LIFE10 NAT/SI/142 PRELIMINARY STUDY OF THE HABITAT, HYDROLOGICAL AND HYDRAULIC CONDITIONS IN THE LJUBLJANICA RIVER CORRIDOR, ESTIMATION OF DANUBE SALMON, DANUBE ROACH AND STRIPED CHUB POPULATION Action: A1 Date: December 2013 1 AN OVERWIEV OF THE RECENT STUDIES ON DANUBE SALMON, DANUBE ROACH AND STRIPED CHUB POPULATION In 2007 dr. Meta Povž and mag. Suzana Šumer took a survey of ecological condition of river Ljubljanica. They monitored the river from the source to the confluence with the Gruber canal in the city of Ljubljana. In addition, historical data about fish on the surveyed part of Ljubljanica were collected. 41 different fish species were registered, among them 31 fish and one lamprey were autochthonous and 9 were aliens. On the basis of historical and acquired data of fish populations they judged that the river Ljubljanica is in moderate ecological status. Ecological potential of the urban river area was not estimated because of lack of proper recent ichthyologic data. 1.1 River Ljubljanica and history of regulations Ljubljanica basin covers an area of 1900 square kilometres what represents almost one tenth of the whole country. The river is 41 kilometres long, which basin is habitat with high diversity, so a real natural museum to comprehend karst secrets, its flora and fauna and human adaptation to nature. Regulation of Ljubljanica started in Roman times, when they were draining and cultivating Ljubljansko Barje and performed regulations of some streams. All the regulations in the 18th and 19th century and in the first half of 20th century were designed for draining and colonising Barje and preventing floods in the city. -

TROTA FARIO Salmo (Trutta) Trutta R.T.I
ASSESSORATO ALL’AGRICOLTURA, CACCIA E PESCA Direzione Agroambiente, Programmazione e Gestione Ittica e Faunistico-Venatoria CARTA ITTICA REGIONALE AI SENSI DELLA DDR N. 188 DEL 23 ottobre 2018 PRESENTAZIONE SULLO STATO DEI POPOLAMENTI SALMONICOLI DEL VENETO Marzo 2020 AQUAPROGRAM srl BIOPROGRAMM soc. Coop. Dr. THOMAS BUSATTO Vicenza Padova - Treviso Venezia www.aquaprogram.it www.bioprogramm.it mandante mandante capogruppo mandataria I SALMONIDI IN ITALIA FAMIGLIA NOMENCLATURA AIIAD (V. CORRENTE) NOME COMUNE ORIGINE Salmonidae Coregonus lavaretus (Linnaeus, 1758) Coregone o Lavarello Alloctona Salmonidae Coregonus oxyrinchus (Linnaeus, 1758) Bondella Alloctona Salmonidae Oncorhynchus kisatch (Walbaum, 1792) Salmone argentato Alloctona Salmonidae Oncorhynchus mykiss (Walbaum, 1792) Trota iridea Alloctona raramente acclimatata Salmonidae Oncorhynchus tshawytscha (Walbaum in Artedi, 1792) Chinook Alloctona non acclimatata Salmonidae Salmo carpio (Linnaeus, 1758) Carpione del Garda Autoctona lago di Garda (endemica) Salmonidae Salmo cettii (Rafinesque, 1810) Trota mediterranea Autoctona Sicilia sud-orientale Salmonidae Salmo fibreni (Zerunian & Gandolfi, 1990) Carpione del Fibreno Autoctona lago Posta Fibreno (endemica) Salmonidae Salmo ghigii (Pomini, 1940) Trota mediterranea Autoctona Appennino e Alpi occidentali Salmonidae Salmo marmoratus (Cuvier, 1829) Trota marmorata Autoctona distretto P.V. (endemica) Salmonidae Salmo trutta (Linnaeus, 1758) Trota fario Alloctona Salmonidae Salmo salar (Linnaeus, 1758) Salmone atlantico Alloctona non -

Trout and Char of Central and Southern Europe and Northern Africa
12 Trout and Char of Central and Southern Europe and Northern Africa Javier Lobón-Cerviá, Manu Esteve, Patrick Berrebi, Antonino Duchi, Massimo Lorenzoni, Kyle A. Young Introduction !e area of central and southern Europe, the Mediterranean, and North Africa spans a wide range of climates from dry deserts to wet forests and temperate maritime to high alpine. !e geologic diversity, glacial history, and long human history of the region have interacted with broad climatic gradients to shape the historical and cur- rent phylogeography of the region’s native trout and char. !e current distributions and abundances of native species are determined in large part by their fundamental niches (i.e., clean, cold water with high dissolved oxygen). Brown Trout Salmo trutta are relatively common and widespread in the northern and mountainous areas of the region but occur in isolated headwater populations in the warmer southern areas of the region. !ese southern areas provided glacial refugia for salmonids and today har- bor much of the region’s phylogenetic diversity. Despite relatively narrow ecologi- cal requirements in terms of water quality, native and invasive trout and char occur throughout the region’s rivers, lakes, estuaries, and coastal waters. Despite having only a single widely recognized native trout species, the region’s range of environments has produced a remarkable diversity of life histories ranging from dwarf, stunted, short and long-lived, small- and large-sized, stream-resident, lake-resident, fluvial potamo- dromous, adfluvial potamodromous, and anadromous (see Chapter 7). Only one trout and one char are native to the region, Brown Trout and Alpine Char Salvelinus umbla. -

Franz Xaver Freiherr Von WULFEN Als Ichthyologe
©Naturwissenschaftlicher Verein für Kärnten, Austria, download unter www.biologiezentrum.at Carinthia II 186./106. Jahrgang S. 349-360 Klagenfurt 1996 Franz Xaver Freiherr von WULFEN als Ichthyologe Von Wolfgang HONSIG-ERLENBURG und Paul MILDNER Mit 5 Abbildungen IN MEMORIAM DR. NORBERT SCHULZ Kurzfassung: In den Beständen der Botanischen Abteilung des Joanneums, Graz, fand sich ein Manuskript zur Fischfauna Kärntens, verfaßt von Franz Xaver Freiherr von WULFEN (1728-1805). Es muß in WULFENS späten Lebensjahren entstanden sein, da u. a. die „Allgemeine Naturgeschich- te der Fische" von M. E. BLOCH, Band 4 (1795) zitiert wird. Behandelt werden folgende Taxa: Salmonidae: Salmo trutta f.fario (LINNE 1758), Salmo trutta f. lacu- stris (LINNE 1758), Salvelinus alpinus salvelinus (LINNE 1758); Coregonidae: Coregonus lavaretus (LINNE 1758)-Formenkreis, Thymallidae: Thymallus tbymallus (LINNE 1758). Bei dem vorliegen- den, hauptsächlich in lateinischer Sprache verfaßten Werk handelt es sich um ein bedeutendes Zeug- nis der autochthonen Fischfauna Kärntens. Abstract: Franz Xaver Freiherr von WULFEN (1728-1805) has written a manuscript about the fishes of Carinthia. This paper was deposed in the Joanneum, Graz. Following taxa were described: Salmonidae: Salmo trutta f. fario (LINNE 1758), Salmo trutta f. lacustris (LINNE 1758), Salvelinus alpi- nus salvelinus (LINNE 1758); Coregonidae: Coregonus lavaretus (LINNE 1758), Thymallidae: Thymal- lus thymallus (LINNE 1758). WULFENS paper, mainly written in Latin language, is a significant report about the original species of fishes that lived in Carinthia. EINLEITUNG • In den Beständen der Botanischen Abteilung des Joanneums in Graz befindet sich ein Manuskript zur Fischfauna Kärntens aus der Feder von Franz Xaver Freiherr von WULFEN (1728-1805). Durch die freundliche Vermittlung von Frau Mag. -

Rediscovery of a Presumed Extinct Species, Salvelinus Profundus, After Re-Oligotrophication
MS. CARMELA J DOENZ (Orcid ID : 0000-0003-4143-7623) Article type : The Scientific Naturalist Running head: The Scientific Naturalist Rediscovery of a presumed extinct species, Salvelinus profundus, after re-oligotrophication Carmela J. Doenz1,2,3, Ole Seehausen1,2 1Department of Fish Ecology & Evolution, EAWAG, Centre for Ecology, Evolution and Biogeochemistry, 6047 Kastanienbaum, Switzerland 2Department of Aquatic Ecology & Evolution, Institute of Ecology and Evolution, University of Bern, 3012 Bern, Switzerland 3Corresponding author. E-mail: [email protected] | downloaded: 13.5.2020 Manuscript received 6 December 2019; revised 29 January 2020; accepted 25 February 2020; final version received 30 March 2020. https://doi.org/10.7892/boris.143521 This article has been accepted for publication and undergone full peer review but has not been throughAccepted Article the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: source: 10.1002/ECY.3065 This article is protected by copyright. All rights reserved Lake Constance (47° 38’ N, 9° 22’ E) is a deep (max. depth 251m) and large (surface area 536 km2) postglacial lake in Central Europe. Originally, it harboured two charr species – Salvelinus umbla and S. profundus. The first is a medium-sized, colourful, winter spawning charr, which is widespread across Central European lakes, the second a small, pale, summer spawning, deepwater charr, which is endemic to Lake Constance (Schillinger 1901, Kottelat and Freyhof 2007). S. profundus has exceptionally large eyes and the upper jaw strongly overlaps the lower jaw. These traits are both considered adaptations to its life in the deep where it mostly feeds on profundal benthos (Schillinger 1901).