Calcareous Sponge Genomes Reveal Complex Evolution of A-Carbonic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spicule Formation in Calcareous Sponges: Coordinated Expression
www.nature.com/scientificreports OPEN Spicule formation in calcareous sponges: Coordinated expression of biomineralization genes and Received: 17 November 2016 Accepted: 02 March 2017 spicule-type specific genes Published: 13 April 2017 Oliver Voigt1, Maja Adamska2, Marcin Adamski2, André Kittelmann1, Lukardis Wencker1 & Gert Wörheide1,3,4 The ability to form mineral structures under biological control is widespread among animals. In several species, specific proteins have been shown to be involved in biomineralization, but it is uncertain how they influence the shape of the growing biomineral and the resulting skeleton. Calcareous sponges are the only sponges that form calcitic spicules, which, based on the number of rays (actines) are distinguished in diactines, triactines and tetractines. Each actine is formed by only two cells, called sclerocytes. Little is known about biomineralization proteins in calcareous sponges, other than that specific carbonic anhydrases (CAs) have been identified, and that uncharacterized Asx-rich proteins have been isolated from calcitic spicules. By RNA-Seq and RNA in situ hybridization (ISH), we identified five additional biomineralization genes inSycon ciliatum: two bicarbonate transporters (BCTs) and three Asx-rich extracellular matrix proteins (ARPs). We show that these biomineralization genes are expressed in a coordinated pattern during spicule formation. Furthermore, two of the ARPs are spicule- type specific for triactines and tetractines (ARP1 orSciTriactinin ) or diactines (ARP2 or SciDiactinin). Our results suggest that spicule formation is controlled by defined temporal and spatial expression of spicule-type specific sets of biomineralization genes. By the process of biomineralization many animal groups produce mineral structures like skeletons, shells and teeth. Biominerals differ in shape considerably from their inorganic mineral counterparts1. -

Associated Organisms Inhabiting the Calcareous Sponge Clathrina Lutea in La Parguera Natural
bioRxiv preprint doi: https://doi.org/10.1101/596429; this version posted April 3, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Associated organisms inhabiting the calcareous sponge Clathrina lutea in La Parguera Natural 2 Reserve, Puerto Rico 3 4 Jaaziel E. García-Hernández1,2*, Nicholas M. Hammerman2,3*, Juan J. Cruz-Motta2 & Nikolaos V. 5 Schizas2 6 * These authors contributed equally 7 1University of Puerto Rico at Mayagüez, Department of Biology, PO Box 9000, Mayagüez, PR 8 00681 9 2University of Puerto Rico at Mayagüez, Department of Marine Sciences, Marine Genomic 10 Biodiversity Laboratory, PO Box 9000, Mayagüez, PR 00681 11 3School of Biological Sciences, University of Queensland, Gehrmann Laboratories, Level 8, 12 Research Road, St Lucia, QLD 4072, Australia 13 14 15 Nikolaos V. Schizas, [email protected], FAX: 787-899-5500 16 17 Running Head: Infauna of the calcareous sponge Clathrina lutea 18 19 20 1 bioRxiv preprint doi: https://doi.org/10.1101/596429; this version posted April 3, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 21 ABSTRACT 22 Sponges provide an array of ecological services and benefits for Caribbean coral reefs. They 23 function as habitats for a bewildering variety of species, however limited attention has been paid 24 in the systematics and distribution of sponge-associated fauna in the class Calcarea or for that 25 matter of sponges in the Caribbean. -

Genomic Insight Into the Host–Endosymbiont Relationship of Endozoicomonas Montiporae CL-33T with Its Coral Host
ORIGINAL RESEARCH published: 08 March 2016 doi: 10.3389/fmicb.2016.00251 Genomic Insight into the Host–Endosymbiont Relationship of Endozoicomonas montiporae CL-33T with its Coral Host Jiun-Yan Ding 1, Jia-Ho Shiu 1, Wen-Ming Chen 2, Yin-Ru Chiang 1 and Sen-Lin Tang 1* 1 Biodiversity Research Center, Academia Sinica, Taipei, Taiwan, 2 Department of Seafood Science, Laboratory of Microbiology, National Kaohsiung Marine University, Kaohsiung, Taiwan The bacterial genus Endozoicomonas was commonly detected in healthy corals in many coral-associated bacteria studies in the past decade. Although, it is likely to be a core member of coral microbiota, little is known about its ecological roles. To decipher potential interactions between bacteria and their coral hosts, we sequenced and investigated the first culturable endozoicomonal bacterium from coral, the E. montiporae CL-33T. Its genome had potential sign of ongoing genome erosion and gene exchange with its Edited by: Rekha Seshadri, host. Testosterone degradation and type III secretion system are commonly present in Department of Energy Joint Genome Endozoicomonas and may have roles to recognize and deliver effectors to their hosts. Institute, USA Moreover, genes of eukaryotic ephrin ligand B2 are present in its genome; presumably, Reviewed by: this bacterium could move into coral cells via endocytosis after binding to coral’s Eph Kathleen M. Morrow, University of New Hampshire, USA receptors. In addition, 7,8-dihydro-8-oxoguanine triphosphatase and isocitrate lyase Jean-Baptiste Raina, are possible type III secretion effectors that might help coral to prevent mitochondrial University of Technology Sydney, Australia dysfunction and promote gluconeogenesis, especially under stress conditions. -

California Marine Life Protection Act (MLPA) Initiative
California Marine Life Protection Act (MLPA) Initiative Evaluation of Existing Central Coast Marine Protected Areas DRAFT, v. 2.0 November 4, 2005 CONTENTS EXECUTIVE SUMMARY ..........................................................................................................................4 1.0 INTRODUCTION ................................................................................................................................8 2.0 EVALUATION OF EXISTING MPAs .................................................................................................11 2.1 Año Nuevo Special Closure...........................................................................................................11 2.2 Elkhorn Slough State Marine Reserve ...........................................................................................12 2.3 Hopkins State Marine Reserve......................................................................................................15 2.4 Pacific Grove State Marine Conservation Area.............................................................................16 2.5: Carmel Bay State Marine Conservation Area ..............................................................................17 2.6 Point Lobos State Marine Reserve................................................................................................19 2.7 Julia Pfeiffer Burns State Marine Conservation Area.....................................................................20 2.8 Big Creek State Marine Reserve....................................................................................................22 -

Review of the Mineralogy of Calcifying Sponges
Dickinson College Dickinson Scholar Faculty and Staff Publications By Year Faculty and Staff Publications 12-2013 Not All Sponges Will Thrive in a High-CO2 Ocean: Review of the Mineralogy of Calcifying Sponges Abigail M. Smith Jade Berman Marcus M. Key, Jr. Dickinson College David J. Winter Follow this and additional works at: https://scholar.dickinson.edu/faculty_publications Part of the Paleontology Commons Recommended Citation Smith, Abigail M.; Berman, Jade; Key,, Marcus M. Jr.; and Winter, David J., "Not All Sponges Will Thrive in a High-CO2 Ocean: Review of the Mineralogy of Calcifying Sponges" (2013). Dickinson College Faculty Publications. Paper 338. https://scholar.dickinson.edu/faculty_publications/338 This article is brought to you for free and open access by Dickinson Scholar. It has been accepted for inclusion by an authorized administrator. For more information, please contact [email protected]. © 2013. Licensed under the Creative Commons http://creativecommons.org/licenses/by- nc-nd/4.0/ Elsevier Editorial System(tm) for Palaeogeography, Palaeoclimatology, Palaeoecology Manuscript Draft Manuscript Number: PALAEO7348R1 Title: Not all sponges will thrive in a high-CO2 ocean: Review of the mineralogy of calcifying sponges Article Type: Research Paper Keywords: sponges; Porifera; ocean acidification; calcite; aragonite; skeletal biomineralogy Corresponding Author: Dr. Abigail M Smith, PhD Corresponding Author's Institution: University of Otago First Author: Abigail M Smith, PhD Order of Authors: Abigail M Smith, PhD; Jade Berman, PhD; Marcus M Key Jr, PhD; David J Winter, PhD Abstract: Most marine sponges precipitate silicate skeletal elements, and it has been predicted that they would be among the few "winners" in an acidifying, high-CO2 ocean. -

Belgian Register of Marine Species
BELGIAN REGISTER OF MARINE SPECIES September 2010 Belgian Register of Marine Species – September 2010 BELGIAN REGISTER OF MARINE SPECIES, COMPILED AND VALIDATED BY THE VLIZ BELGIAN MARINE SPECIES CONSORTIUM VLIZ SPECIAL PUBLICATION 46 SUGGESTED CITATION Leen Vandepitte, Wim Decock & Jan Mees (eds) (2010). Belgian Register of Marine Species, compiled and validated by the VLIZ Belgian Marine Species Consortium. VLIZ Special Publication, 46. Vlaams Instituut voor de Zee (VLIZ): Oostende, Belgium. 78 pp. ISBN 978‐90‐812900‐8‐1. CONTACT INFORMATION Flanders Marine Institute – VLIZ InnovOcean site Wandelaarkaai 7 8400 Oostende Belgium Phone: ++32‐(0)59‐34 21 30 Fax: ++32‐(0)59‐34 21 31 E‐mail: [email protected] or [email protected] ‐ 2 ‐ Belgian Register of Marine Species – September 2010 Content Introduction ......................................................................................................................................... ‐ 5 ‐ Used terminology and definitions ....................................................................................................... ‐ 7 ‐ Belgian Register of Marine Species in numbers .................................................................................. ‐ 9 ‐ Belgian Register of Marine Species ................................................................................................... ‐ 12 ‐ BACTERIA ............................................................................................................................................. ‐ 12 ‐ PROTOZOA ........................................................................................................................................... -

Porifera) in Singapore and Description of a New Species of Forcepia (Poecilosclerida: Coelosphaeridae)
Contributions to Zoology, 81 (1) 55-71 (2012) Biodiversity of shallow-water sponges (Porifera) in Singapore and description of a new species of Forcepia (Poecilosclerida: Coelosphaeridae) Swee-Cheng Lim1, 3, Nicole J. de Voogd2, Koh-Siang Tan1 1 Tropical Marine Science Institute, National University of Singapore, 18 Kent Ridge Road, Singapore 119227, Singapore 2 Netherlands Centre for Biodiversity, Naturalis, PO Box 9517, 2300 RA Leiden, The Netherlands 3 E-mail: [email protected] Key words: intertidal, Southeast Asia, sponge assemblage, subtidal, tropical Abstract gia) patera (Hardwicke, 1822) was the first sponge de- scribed from Singapore in the 19th century. This was A surprisingly high number of shallow water sponge species followed by Leucosolenia flexilis (Haeckel, 1872), (197) were recorded from extensive sampling of natural inter- Coelocarteria singaporensis (Carter, 1883) (as Phloeo tidal and subtidal habitats in Singapore (Southeast Asia) from May 2003 to June 2010. This is in spite of a highly modified dictyon), and Callyspongia (Cladochalina) diffusa coastline that encompasses one of the world’s largest container Ridley (1884). Subsequently, Dragnewitsch (1906) re- ports as well as extensive oil refining and bunkering industries. corded 24 sponge species from Tanjong Pagar and Pu- A total of 99 intertidal species was recorded in this study. Of lau Brani in the Singapore Strait. A further six species these, 53 species were recorded exclusively from the intertidal of sponge were reported from Singapore in the 1900s, zone and only 45 species were found on both intertidal and subtidal habitats, suggesting that tropical intertidal and subtidal although two species, namely Cinachyrella globulosa sponge assemblages are different and distinct. -

Xenacoelomorpha's Significance for Understanding Bilaterian Evolution
Available online at www.sciencedirect.com ScienceDirect Xenacoelomorpha’s significance for understanding bilaterian evolution Andreas Hejnol and Kevin Pang The Xenacoelomorpha, with its phylogenetic position as sister biology models are the fruitfly Drosophila melanogaster and group of the Nephrozoa (Protostomia + Deuterostomia), plays the nematode Caenorhabditis elegans, in which basic prin- a key-role in understanding the evolution of bilaterian cell types ciples of developmental processes have been studied in and organ systems. Current studies of the morphological and great detail. It might be because the field of evolutionary developmental diversity of this group allow us to trace the developmental biology — EvoDevo — has its origin in evolution of different organ systems within the group and to developmental biology and not evolutionary biology that reconstruct characters of the most recent common ancestor of species under investigation are often called ‘model spe- Xenacoelomorpha. The disparity of the clade shows that there cies’. Criteria for selected representative species are cannot be a single xenacoelomorph ‘model’ species and primarily the ease of access to collected material and strategic sampling is essential for understanding the evolution their ability to be cultivated in the lab [1]. In some cases, of major traits. With this strategy, fundamental insights into the a supposedly larger number of ancestral characters or a evolution of molecular mechanisms and their role in shaping dominant role in ecosystems have played an additional animal organ systems can be expected in the near future. role in selecting model species. These arguments were Address used to attract sufficient funding for genome sequencing Sars International Centre for Marine Molecular Biology, University of and developmental studies that are cost-intensive inves- Bergen, Thormøhlensgate 55, 5008 Bergen, Norway tigations. -

A New Species of the Calcareous Sponge Genus Leuclathrina (Calcarea: Calcinea: Clathrinida) from the Maldives
Zootaxa 4382 (1): 147–158 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2018 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4382.1.5 http://zoobank.org/urn:lsid:zoobank.org:pub:B222C2D8-82FB-414C-A88F-44A12A837A21 A new species of the calcareous sponge genus Leuclathrina (Calcarea: Calcinea: Clathrinida) from the Maldives OLIVER VOIGT1,5, BERNHARD RUTHENSTEINER2, LAURA LEIVA1, BENEDETTA FRADUSCO1 & GERT WÖRHEIDE1,3,4 1Department of Earth and Environmental Sciences, Palaeontology and Geobiology, Ludwig-Maximilians-Universität München, Rich- ard-Wagner-Str. 10, 80333 München, Germany 2 SNSB - Zoologische Staatssammlung München, Sektion Evertebrata varia, Münchhausenstr. 21, 81247 München, Germany 3 GeoBio-Center, Ludwig-Maximilians-Universität München, Richard-Wagner-Str. 10, 80333 München, Germany 4SNSB - Bayerische Staatssammlung für Paläontologie und Geologie, Richard-Wagner-Str. 10, 80333 München, Germany 5Corresponding author. E-mail: [email protected], Tel.: +49 (0) 89 2180 6635; Fax: +49 (0) 89 2180 6601 Abstract The diversity and phylogenetic relationships of calcareous sponges are still not completely understood. Recent integrative approaches combined analyses of DNA and morphological observations. Such studies resulted in severe taxonomic revi- sions within the subclass Calcinea and provided the foundation for a phylogenetically meaningful classification. However, several genera are missing from DNA phylogenies and their relationship to other Calcinea remain uncertain. One of these genera is Leuclathrina (family Leucaltidae). We here describe a new species from the Maldives, Leuclathrina translucida sp. nov., which is only the second species of the genus. Like the type species Leuclathrina asconoides, the new species has a leuconoid aquiferous system and lacks a specialized choanoskeleton. -

Animal Evolution: Looking for the First Nervous System
Dispatch R655 cellular or symbiotic functions, cannot genome reduction in the symbiont, 10. Nakabachi, A., Ishida, K., Hongoh, Y., Ohkuma, M., and Miyagishima, S. (2014). be lost without harmful or fatal results. HGT from various sources to the host Aphid gene of bacterial origin encodes a But these genes can be replaced, genome to maintain symbiont function, protein transported to an obligate and perhaps ever more easily as and now the targeting of protein endosymbiont. Curr. Biol. 24, R640–R641. 11. Moran, N.A., and Jarvik, T. (2010). Lateral the interaction networks of the products from host to symbiont has transfer of genes from fungi underlies endosymbiont reduce in complexity, even been found [4,10]. These make carotenoid production in aphids. Science 328, 624–627. thus reducing pressures on proteins clean separation of endosymbiont from 12. Jorgenson, M.A., Chen, Y., Yahashiri, A., to co-evolve. In a sense, when genes organelle more difficult to see, Popham, D.L., and Weiss, D.S. (2014). The are transferred from symbiont or prompting us not to look for the point bacterial septal ring protein RlpA is a lytic transglycosylase that contributes to rod organelle to the host nuclear genome when a symbiont ‘becomes’ an shape and daughter cell separation in and their proteins targeted back, this is organelle, but rather to ask, ‘Is there Pseudomonas aeruginosa. Mol. Microbiol. 93, 113–128. a compensatory change [4,9,14,17]. really anything so special about 13. Acuna, R., Padilla, B.E., Florez-Ramos, C.P., But other compensatory transfers that organelles?’ Rubio, J.D., Herrera, J.C., Benavides, P., affect a symbiont or organelle can also Lee, S.J., Yeats, T.H., Egan, A.N., Doyle, J.J., et al. -

Carbonic Anhydrases: an Ancient Tool in Calcareous Sponge Biomineralization
fgene-12-624533 March 30, 2021 Time: 13:30 # 1 BRIEF RESEARCH REPORT published: 07 April 2021 doi: 10.3389/fgene.2021.624533 Carbonic Anhydrases: An Ancient Tool in Calcareous Sponge Biomineralization Oliver Voigt1*, Benedetta Fradusco1, Carolin Gut1, Charalampos Kevrekidis1, Sergio Vargas1 and Gert Wörheide1,2,3 1 Department of Earth and Environmental Sciences, Palaeontology and Geobiology, Ludwig-Maximilians-Universität München, Munich, Germany, 2 GeoBio-Center, Ludwig-Maximilians-Universität München, Munich, Germany, 3 SNSB-Bayerische Staatssammlung für Paläontologie und Geologie, Munich, Germany Enzymes of the a-carbonic anhydrase gene family (CAs) are essential for the deposition of calcium carbonate biominerals. In calcareous sponges (phylum Porifera, class Calcarea), specific CAs are involved in the formation of calcite spicules, a unique trait and synapomorphy of this class. However, detailed studies on the CA repertoire of calcareous sponges exist for only two species of one of the two Calcarea subclasses, the Calcaronea. The CA repertoire of the second subclass, the Calcinea, has not been investigated so far, leaving a considerable gap in our knowledge about this Edited by: Melanie Debiais-Thibaud, gene family in Calcarea. Here, using transcriptomic analysis, phylogenetics, and in situ Université de Montpellier, France hybridization, we study the CA repertoire of four additional species of calcareous Reviewed by: sponges, including three from the previously unsampled subclass Calcinea. Our data Helena Cetkovi´ c,´ Rudjer Boskovic Institute, Croatia indicate that the last common ancestor of Calcarea had four ancestral CAs with defined Ana Riesgo, subcellular localizations and functions (mitochondrial/cytosolic, membrane-bound, and Natural History Museum, secreted non-catalytic). The evolution of membrane-bound and secreted CAs involved United Kingdom gene duplications and losses, whereas mitochondrial/cytosolic and non-catalytic CAs *Correspondence: Oliver Voigt are evidently orthologous genes. -
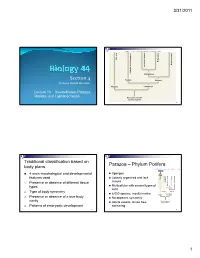
S I Section 4
3/31/2011 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Porifera Ecdysozoa Deuterostomia Lophotrochozoa Cnidaria and Ctenophora Cnidaria and Protostomia SSiection 4 Radiata Bilateria Professor Donald McFarlane Parazoa Eumetazoa Lecture 13 Invertebrates: Parazoa, Radiata, and Lophotrochozoa Ancestral colonial choanoflagellate 2 Traditional classification based on Parazoa – Phylum Porifera body plans Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Parazoa 4 main morphological and developmental Sponges features used Loosely organized and lack Porifera Ecdysozoa Cnidaria and Ctenophora tissues euterostomia photrochozoa D 1. o Presence or absence of different tissue L Multicellular with several types of types Protostomia cells 2. Type of body symmetry Radiata Bilateria 8,000 species, mostly marine Parazoa Eumetazoa 3. Presence or absence of a true body No apparent symmetry cavity Ancestral colonial Adults sessile, larvae free- choanoflagellate 4. Patterns of embryonic development swimming 3 4 1 3/31/2011 Water drawn through pores (ostia) into spongocoel Flows out through osculum Reproduce Choanocytes line spongocoel Sexually Most hermapppgggphrodites producing eggs and sperm Trap and eat small particles and plankton Gametes are derived from amoebocytes or Mesohyl between choanocytes and choanocytes epithelial cells Asexually Amoebocytes absorb food from choanocytes, Small fragment or bud may detach and form a new digest it, and carry