Poaceae: Pooideae: Stipeae) Based on Analysis of Multiple Chloroplast Loci, ITS, and Lemma Micromorphology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Taxonomical Notes on the Genus Piptatherum P. Beauv
TAXONOMICAL NOTES ON THE GENUS PIPTATHERUM P. BEAUV. (POACEAE) IN IRAN ee & M. Assadi׳B. Hamzeh Received 2015. 02. 20; accepted for publication 2015. 05. 12 ee, B. & Assadi, M. 2015. 06. 30: Taxonomical notes on the genus Piptatherum P. Beauv. (Poaceae) in׳Hamzeh Iran. –Iran. J. Bot. 21 (1): 01-09. Tehran. Piptatherum denaense is described as a new species from south west of Iran, Dena Mountain. It is close to P. laterale but differs from it by having glabrous lemma in lower half and toward the apex, narrower vegetative shoots and unbearded anthers. Piptatherum holciforme subsp. holciforme var. glabrum is not accepted as a distinct variety. Piptatherum sphacelatum formerly known as a synonym of P. molinioides is established as a separate species due to having distinct morphological characters as well as molecular differences based on literature. Thus, the number of taxa in the genus Piptatherum changes to 9 species, 2 subspecies and 4 varieties in Iran. An identification key to Piptatherum taxa occuring in Iran is provided. The distribution map of P. denaense with P. laterale subsp. laterale and the illustration of the new species are included. ee (correspondence <[email protected]>) & Mostafa Assadi, Research Institute of Forests and׳Behnam Hamzeh Rangelands, P. O. Box: 13185-116, Tehran, Iran. Key words: Piptatherum; Poaceae; new species; new synonym; reestablished species; Iran ﻧﻜﺎﺗﻲ در ﻣﻮرد ﺟﻨﺲ .Poaceae ) Piptatherum P. Beauv) در اﻳﺮان ﺑﻬﻨﺎم ﺣﻤﺰة، اﺳﺘﺎدﻳﺎر ﭘﮋوﻫﺶ ﻣﺆﺳﺴﻪ ﺗﺤﻘﻴﻘﺎت ﺟﻨﮕﻠﻬﺎ و ﻣﺮاﺗﻊ ﻛﺸﻮر ﻣﺼﻄﻔﻲ اﺳﺪي، اﺳﺘﺎد ﭘﮋوﻫﺶ ﻣﺆﺳﺴﻪ ﺗﺤﻘﻴﻘﺎت ﺟﻨﮕﻠﻬﺎ و ﻣﺮاﺗﻊ ﻛﺸﻮر Piptatherum denaense ﺑﻪﻋﻨﻮان ﮔﻮﻧﻪاي ﺟﺪﻳﺪ از ﻛﻮه دﻧﺎ در ﺟﻨﻮب ﻏﺮب اﻳﺮان ﺷﺮح داده ﻣﻲﺷﻮد. -
A REVISION of TRISETUM Victor L. Finot,' Paul M
A REVISION OF TRISETUM Victor L. Finot,' Paul M. Peterson,3 (POACEAE: POOIDEAE: Fernando 0 Zuloaga,* Robert J. v sorene, and Oscar Mattnei AVENINAE) IN SOUTH AMERICA1 ABSTRACT A taxonomic treatment of Trisetum Pers. for South America, is given. Eighteen species and six varieties of Trisetum are recognized in South America. Chile (14 species, 3 varieties) and Argentina (12 species, 5 varieties) have the greatest number of taxa in the genus. Two varieties, T. barbinode var. sclerophyllum and T longiglume var. glabratum, are endemic to Argentina, whereas T. mattheii and T nancaguense are known only from Chile. Trisetum andinum is endemic to Ecuador, T. macbridei is endemic to Peru, and T. foliosum is endemic to Venezuela. A total of four species are found in Ecuador and Peru, and there are two species in Venezuela and Colombia. The following new species are described and illustrated: Trisetum mattheii Finot and T nancaguense Finot, from Chile, and T pyramidatum Louis- Marie ex Finot, from Chile and Argentina. The following two new combinations are made: T barbinode var. sclerophyllum (Hack, ex Stuck.) Finot and T. spicatum var. cumingii (Nees ex Steud.) Finot. A key for distinguishing the species and varieties of Trisetum in South America is given. The names Koeleria cumingii Nees ex Steud., Trisetum sect. Anaulacoa Louis-Marie, Trisetum sect. Aulacoa Louis-Marie, Trisetum subg. Heterolytrum Louis-Marie, Trisetum subg. Isolytrum Louis-Marie, Trisetum subsect. Koeleriformia Louis-Marie, Trisetum subsect. Sphenopholidea Louis-Marie, Trisetum ma- lacophyllum Steud., Trisetum variabile E. Desv., and Trisetum variabile var. virescens E. Desv. are lectotypified. Key words: Aveninae, Gramineae, Poaceae, Pooideae, Trisetum. -

The Vegetation of Robinson Crusoe Island (Isla Masatierra), Juan
The Vegetation ofRobinson Crusoe Island (Isla Masatierra), Juan Fernandez Archipelago, Chile1 Josef Greimler,2,3 Patricio Lopez 5., 4 Tod F. Stuessy, 2and Thomas Dirnbiick5 Abstract: Robinson Crusoe Island of the Juan Fernandez Archipelago, as is the case with many oceanic islands, has experienced strong human disturbances through exploitation ofresources and introduction of alien biota. To understand these impacts and for purposes of diversity and resource management, an accu rate assessment of the composition and structure of plant communities was made. We analyzed the vegetation with 106 releves (vegetation records) and subsequent Twinspan ordination and produced a detailed colored map at 1: 30,000. The resultant map units are (1) endemic upper montane forest, (2) endemic lower montane forest, (3) Ugni molinae shrubland, (4) Rubus ulmifolius Aristotelia chilensis shrubland, (5) fern assemblages, (6) Libertia chilensis assem blage, (7) Acaena argentea assemblage, (8) native grassland, (9) weed assemblages, (10) tall ruderals, and (11) cultivated Eucalyptus, Cupressus, and Pinus. Mosaic patterns consisting of several communities are recognized as mixed units: (12) combined upper and lower montane endemic forest with aliens, (13) scattered native vegetation among rocks at higher elevations, (14) scattered grassland and weeds among rocks at lower elevations, and (15) grassland with Acaena argentea. Two categories are included that are not vegetation units: (16) rocks and eroded areas, and (17) settlement and airfield. Endemic forests at lower elevations and in drier zones of the island are under strong pressure from three woody species, Aristotelia chilensis, Rubus ulmifolius, and Ugni molinae. The latter invades native forests by ascending dry slopes and ridges. -
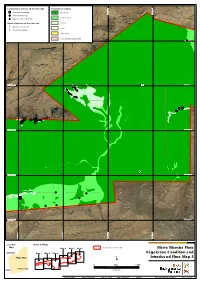
Additional Information
Current Survey Introduced Flora Records Vegetation Condition *Acetosa vesicaria Excellent 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 -

Types of American Grasses
z LIBRARY OF Si AS-HITCHCOCK AND AGNES'CHASE 4: SMITHSONIAN INSTITUTION UNITED STATES NATIONAL MUSEUM oL TiiC. CONTRIBUTIONS FROM THE United States National Herbarium Volume XII, Part 3 TXE&3 OF AMERICAN GRASSES . / A STUDY OF THE AMERICAN SPECIES OF GRASSES DESCRIBED BY LINNAEUS, GRONOVIUS, SLOANE, SWARTZ, AND MICHAUX By A. S. HITCHCOCK z rit erV ^-C?^ 1 " WASHINGTON GOVERNMENT PRINTING OFFICE 1908 BULLETIN OF THE UNITED STATES NATIONAL MUSEUM Issued June 18, 1908 ii PREFACE The accompanying paper, by Prof. A. S. Hitchcock, Systematic Agrostologist of the United States Department of Agriculture, u entitled Types of American grasses: a study of the American species of grasses described by Linnaeus, Gronovius, Sloane, Swartz, and Michaux," is an important contribution to our knowledge of American grasses. It is regarded as of fundamental importance in the critical sys- tematic investigation of any group of plants that the identity of the species described by earlier authors be determined with certainty. Often this identification can be made only by examining the type specimen, the original description being inconclusive. Under the American code of botanical nomenclature, which has been followed by the author of this paper, "the nomenclatorial t}rpe of a species or subspecies is the specimen to which the describer originally applied the name in publication." The procedure indicated by the American code, namely, to appeal to the type specimen when the original description is insufficient to identify the species, has been much misunderstood by European botanists. It has been taken to mean, in the case of the Linnsean herbarium, for example, that a specimen in that herbarium bearing the same name as a species described by Linnaeus in his Species Plantarum must be taken as the type of that species regardless of all other considerations. -

Wiregrass (Aristida Stricta)
Wiregrass (Aristida stricta) For definitions of botanical terms, visit en.wikipedia.org/wiki/Glossary_of_botanical_terms. Wiregrass is a perennial bunchgrass found in scrub, pinelands and coastal uplands throughout much of Florida. It is the dominant groundcover species in longleaf pine savannas and is a primary food source for gopher tortoises. Birds and small wildlife eat the seeds. Historically, cattle grazed on Wiregrass’s tender new growth. Wiregrass flowers are tiny and brown. They are born on spikelike terminal panicles Flower stalks are elongated and extend above the leaves. Leaf blades are long, thin and rolled inward, giving them a wiry appearance (hence the common name). They are erect and green when young Photo courtesy of Alan Cressler, Lady Bird Johnson Wildflower Center and begin to arch and turn brown as they age. Its fruits are small, yellowish caryopses. Seeds may be dispersed by wind, gravity or on the fur of passing animals. The genus name Aristida is from the Latin arista, meaning “awn” and referring to the three awns or bristle-like structures that extend from the florets. (An alternative common name is Pineland threeawn.) The species epithet, stricta, is from the Latin strictus, meaning straight or erect. Family: Poaceae (Grass family) Native range: Nearly throughout To see where natural populations of Wiregrass have been vouchered, visit www.florida.plantatlas.usf.edu. Hardiness: Zones 8A–10B Lifespan: Perennial Soil: Moist to dry, well-drained sandy soils Exposure: Full sun to partial shade Growth habit: 1–3’+ tall and equally wide Propagation: Division, seed Garden tips: Wiregrass is fast-growing and tolerant of drought conditions and low-nutrient soils. -

Project Report
THE APPLICATION OF PHYTOLITH AND STARCH GRAIN ANALYSIS TO UNDERSTANDING FORMATIVE PERIOD SUBSISTENCE, RITUAL, AND TRADE ON THE TARACO PENINSULA, HIGHLAND BOLIVIA ___________________________________________________________________ A Thesis Presented to the Faculty of the Graduate School University of Missouri, Columbia ___________________________________________________________________ In Partial Fulfillment Of the Requirements for the Degree Master of Arts ___________________________________________________________________ By AMANDA LEE LOGAN Supervisor: Dr. Deborah M. Pearsall AUGUST 2006 Dedicated to the memory of my grandmother Joanne Marie Higgins 1940-2005 ACKNOWLEDGEMENTS There are a great number of people who have helped in this process in passing or in long, detailed conversations, and everything in between. First and foremost, many thanks to my advisor, Debby Pearsall, for creative and inspired guidance, and for taking the time to talk over everything from the smallest detail to the biggest challenges. Debby introduced me to the world of phytoliths, and then to the wonders of starch grains, and encouraged me to find and pursue the issues that drive me. My committee has been very helpful and patient, and made my oral exams and defense far more enjoyable then expected—Dr. Christine Hastorf, Dr. Bob Benfer, and Dr. Randy Miles. Dr. Benfer was crucial in helping me sort through the statistical applications. I also benefited tremendously from conversations with and advice from my cohorts in the MU Paleoethnobotany lab, or as we are better known, the “Pearsall Youth”— Neil Duncan, Shawn Collins, Meghann O’Brien, Tom Hart, and Nicole Little. Dr. Karol Chandler-Ezell gave me great advice on calcium oxalate and chemical processing. Dr. Todd VanPool graciously provided much needed advice on the statistical applications. -

BFS048 Site Species List
Species lists based on plot records from DEP (1996), Gibson et al. (1994), Griffin (1993), Keighery (1996) and Weston et al. (1992). Taxonomy and species attributes according to Keighery et al. (2006) as of 16th May 2005. Species Name Common Name Family Major Plant Group Significant Species Endemic Growth Form Code Growth Form Life Form Life Form - aquatics Common SSCP Wetland Species BFS No kens01 (FCT23a) Wd? Acacia sessilis Wattle Mimosaceae Dicot WA 3 SH P 48 y Acacia stenoptera Narrow-winged Wattle Mimosaceae Dicot WA 3 SH P 48 y * Aira caryophyllea Silvery Hairgrass Poaceae Monocot 5 G A 48 y Alexgeorgea nitens Alexgeorgea Restionaceae Monocot WA 6 S-R P 48 y Allocasuarina humilis Dwarf Sheoak Casuarinaceae Dicot WA 3 SH P 48 y Amphipogon turbinatus Amphipogon Poaceae Monocot WA 5 G P 48 y * Anagallis arvensis Pimpernel Primulaceae Dicot 4 H A 48 y Austrostipa compressa Golden Speargrass Poaceae Monocot WA 5 G P 48 y Banksia menziesii Firewood Banksia Proteaceae Dicot WA 1 T P 48 y Bossiaea eriocarpa Common Bossiaea Papilionaceae Dicot WA 3 SH P 48 y * Briza maxima Blowfly Grass Poaceae Monocot 5 G A 48 y Burchardia congesta Kara Colchicaceae Monocot WA 4 H PAB 48 y Calectasia narragara Blue Tinsel Lily Dasypogonaceae Monocot WA 4 H-SH P 48 y Calytrix angulata Yellow Starflower Myrtaceae Dicot WA 3 SH P 48 y Centrolepis drummondiana Sand Centrolepis Centrolepidaceae Monocot AUST 6 S-C A 48 y Conostephium pendulum Pearlflower Epacridaceae Dicot WA 3 SH P 48 y Conostylis aculeata Prickly Conostylis Haemodoraceae Monocot WA 4 H P 48 y Conostylis juncea Conostylis Haemodoraceae Monocot WA 4 H P 48 y Conostylis setigera subsp. -

Bio 308-Course Guide
COURSE GUIDE BIO 308 BIOGEOGRAPHY Course Team Dr. Kelechi L. Njoku (Course Developer/Writer) Professor A. Adebanjo (Programme Leader)- NOUN Abiodun E. Adams (Course Coordinator)-NOUN NATIONAL OPEN UNIVERSITY OF NIGERIA BIO 308 COURSE GUIDE National Open University of Nigeria Headquarters 14/16 Ahmadu Bello Way Victoria Island Lagos Abuja Office No. 5 Dar es Salaam Street Off Aminu Kano Crescent Wuse II, Abuja e-mail: [email protected] URL: www.nou.edu.ng Published by National Open University of Nigeria Printed 2013 ISBN: 978-058-434-X All Rights Reserved Printed by: ii BIO 308 COURSE GUIDE CONTENTS PAGE Introduction ……………………………………......................... iv What you will Learn from this Course …………………............ iv Course Aims ……………………………………………............ iv Course Objectives …………………………………………....... iv Working through this Course …………………………….......... v Course Materials ………………………………………….......... v Study Units ………………………………………………......... v Textbooks and References ………………………………........... vi Assessment ……………………………………………….......... vi End of Course Examination and Grading..................................... vi Course Marking Scheme................................................................ vii Presentation Schedule.................................................................... vii Tutor-Marked Assignment ……………………………….......... vii Tutors and Tutorials....................................................................... viii iii BIO 308 COURSE GUIDE INTRODUCTION BIO 308: Biogeography is a one-semester, 2 credit- hour course in Biology. It is a 300 level, second semester undergraduate course offered to students admitted in the School of Science and Technology, School of Education who are offering Biology or related programmes. The course guide tells you briefly what the course is all about, what course materials you will be using and how you can work your way through these materials. It gives you some guidance on your Tutor- Marked Assignments. There are Self-Assessment Exercises within the body of a unit and/or at the end of each unit. -

Department of the Interior Fish and Wildlife Service
Tuesday, February 10, 2009 Part II Department of the Interior Fish and Wildlife Service 50 CFR Part 17 Endangered and Threatened Wildlife and Plants; Determination of Endangered Status for Reticulated Flatwoods Salamander; Designation of Critical Habitat for Frosted Flatwoods Salamander and Reticulated Flatwoods Salamander; Final Rule VerDate Nov<24>2008 14:17 Feb 09, 2009 Jkt 217001 PO 00000 Frm 00001 Fmt 4717 Sfmt 4717 E:\FR\FM\10FER2.SGM 10FER2 erowe on PROD1PC63 with RULES_2 6700 Federal Register / Vol. 74, No. 26 / Tuesday, February 10, 2009 / Rules and Regulations DEPARTMENT OF THE INTERIOR during normal business hours, at U.S. Register on or before July 30, 2008, with Fish and Wildlife Service, Mississippi the final critical habitat rule to be Fish and Wildlife Service Fish and Wildlife Office, 6578 Dogwood submitted for publication in the Federal View Parkway, Jackson, MS 39213. Register by January 30, 2009. The 50 CFR Part 17 FOR FURTHER INFORMATION CONTACT: Ray revised proposed rule was signed on [FWS–R4–ES–2008–0082; MO 9921050083– Aycock, Field Supervisor, U.S. Fish and and delivered to the Federal Register on B2] Wildlife Service, Mississippi Field July 30, 2008, and it subsequently Office, 6578 Dogwood View Parkway, published on August 13, 2008 (73 FR RIN 1018–AU85 Jackson, MS 39213; telephone: 601– 47258). We also published supplemental information on the Endangered and Threatened Wildlife 321–1122; facsimile: 601–965–4340. If proposed rule to maintain the status of and Plants; Determination of you use a telecommunications device the frosted flatwoods salamander as Endangered Status for Reticulated for the deaf (TDD), call the Federal Information Relay Service (FIRS) at threatened (73 FR 54125; September 18, Flatwoods Salamander; Designation of 2008). -

Time Lag Between Glacial Retreat and Upward Migration Alters Tropical Alpine ☆ T Communities
Perspectives in Plant Ecology, Evolution and Systematics 30 (2018) 89–102 Contents lists available at ScienceDirect Perspectives in Plant Ecology, Evolution and Systematics journal homepage: www.elsevier.com/locate/ppees Research article Time lag between glacial retreat and upward migration alters tropical alpine ☆ T communities Anaïs Zimmera,b,c, Rosa I. Menesesb, Antoine Rabateld, Alvaro Sorucoe, Olivier Danglesf,g,h, ⁎ Fabien Anthelmea,b,c, a AMAP, IRD, CNRS, INRA, Université de Montpellier, Montpellier, France b Museo Nacional de Historia Natural, Herbario Nacional de Bolivia (LPB), Cota Cota, Casilla 10077 Correo Central, La Paz, Bolivia c Instituto de Ecología, Universidad Mayor San Andrés, Calle 27, Cota Cota, Campus Universitario, La Paz, Bolivia d Université Grenoble Alpes, CNRS, IRD, Institut des Géosciences de l’Environnement (IGE, UMR 5001), F-38000 Grenoble, France e Instituto de Geología y del Medio Ambiente, Universidad Mayor San Andrés, Calle 27, Cota Cota, Campus Universitario, La Paz, Bolivia f Institut de Recherche pour le Développement (IRD), EGCE, 91198 Gif-sur-Yvette Cedex, France g Université Paris-Sud 11, 91405 Orsay Cedex, France h Pontificia Universidad Católica del Ecuador, Facultad de Ciencias Exactas y Naturales, Quito, Ecuador ARTICLE INFO ABSTRACT Keywords: Species range shifts and possible species extinctions in alpine regions are hypothesized being influenced by the Biological soil crust increasing time lag between the velocity of global warming and the slowness of primary succession. We tested this Climatic debt hypothesis in tropical alpine environments above 4700 m a.s.l. (Central Andes) and we explored the underlying Chronosequence mechanisms at work by using four sites gradually deglaciated since the acceleration of warming in the late 1970’s. -

Poaceae: Pooideae) Based on Plastid and Nuclear DNA Sequences
d i v e r s i t y , p h y l o g e n y , a n d e v o l u t i o n i n t h e monocotyledons e d i t e d b y s e b e r g , p e t e r s e n , b a r f o d & d a v i s a a r h u s u n i v e r s i t y p r e s s , d e n m a r k , 2 0 1 0 Phylogenetics of Stipeae (Poaceae: Pooideae) Based on Plastid and Nuclear DNA Sequences Konstantin Romaschenko,1 Paul M. Peterson,2 Robert J. Soreng,2 Núria Garcia-Jacas,3 and Alfonso Susanna3 1M. G. Kholodny Institute of Botany, Tereshchenkovska 2, 01601 Kiev, Ukraine 2Smithsonian Institution, Department of Botany MRC-166, National Museum of Natural History, P.O. Box 37012, Washington, District of Columbia 20013-7012 USA. 3Laboratory of Molecular Systematics, Botanic Institute of Barcelona (CSIC-ICUB), Pg. del Migdia, s.n., E08038 Barcelona, Spain Author for correspondence ([email protected]) Abstract—The Stipeae tribe is a group of 400−600 grass species of worldwide distribution that are currently placed in 21 genera. The ‘needlegrasses’ are char- acterized by having single-flowered spikelets and stout, terminally-awned lem- mas. We conducted a molecular phylogenetic study of the Stipeae (including all genera except Anemanthele) using a total of 94 species (nine species were used as outgroups) based on five plastid DNA regions (trnK-5’matK, matK, trnHGUG-psbA, trnL5’-trnF, and ndhF) and a single nuclear DNA region (ITS).