Hd& Safety Data Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exploring Density
Exploring Density Students investigate the densities of different liquids and solids and understand how density may help identify a substance. Suggested Grade Range: 6-8 Approximate Time: 1 hour Relevant National Content Standards: Next Generation Science Standards Science and Engineering Practices: Developing and using Models Modeling in 6–8 builds on K–5 experiences and progresses to developing, using, and revising models to describe, test, and predict more abstract phenomena and design systems. • Develop and use a model to describe phenomena. Science and Engineering Practices: Analyzing and Interpreting Data Analyzing data in 6-8 builds on K-5 and progresses to extending quantitative analysis to investigations, distinguishing between correlation and causation, and basic statistical techniques of data and error analysis. • Analyze and interpret data to determine similarities and differences in findings. Disciplinary Core Ideas: PS1.A Structure and Properties of Matter Each pure substance has characteristic physical and chemical properties (for any bulk quantity under given conditions) that can be used to identify it. Common Core State Standard: 7NS 2. Apply and extend previous understanding of multiplication and division and of fractions to multiply and divide rational numbers. 3. Solve real-world and mathematical problems involving the four operations with rational numbers. Common Core State Standard: 7EE Solve real-life and mathematical problems using numerical and algebraic expressions and equations. 4. Use variables to represent -

Lecture Notes
Solid State Physics PHYS 40352 by Mike Godfrey Spring 2012 Last changed on May 22, 2017 ii Contents Preface v 1 Crystal structure 1 1.1 Lattice and basis . .1 1.1.1 Unit cells . .2 1.1.2 Crystal symmetry . .3 1.1.3 Two-dimensional lattices . .4 1.1.4 Three-dimensional lattices . .7 1.1.5 Some cubic crystal structures ................................ 10 1.2 X-ray crystallography . 11 1.2.1 Diffraction by a crystal . 11 1.2.2 The reciprocal lattice . 12 1.2.3 Reciprocal lattice vectors and lattice planes . 13 1.2.4 The Bragg construction . 14 1.2.5 Structure factor . 15 1.2.6 Further geometry of diffraction . 17 2 Electrons in crystals 19 2.1 Summary of free-electron theory, etc. 19 2.2 Electrons in a periodic potential . 19 2.2.1 Bloch’s theorem . 19 2.2.2 Brillouin zones . 21 2.2.3 Schrodinger’s¨ equation in k-space . 22 2.2.4 Weak periodic potential: Nearly-free electrons . 23 2.2.5 Metals and insulators . 25 2.2.6 Band overlap in a nearly-free-electron divalent metal . 26 2.2.7 Tight-binding method . 29 2.3 Semiclassical dynamics of Bloch electrons . 32 2.3.1 Electron velocities . 33 2.3.2 Motion in an applied field . 33 2.3.3 Effective mass of an electron . 34 2.4 Free-electron bands and crystal structure . 35 2.4.1 Construction of the reciprocal lattice for FCC . 35 2.4.2 Group IV elements: Jones theory . 36 2.4.3 Binding energy of metals . -

Glossary of Terms
GLOSSARY OF TERMS For the purpose of this Handbook, the following definitions and abbreviations shall apply. Although all of the definitions and abbreviations listed below may have not been used in this Handbook, the additional terminology is provided to assist the user of Handbook in understanding technical terminology associated with Drainage Improvement Projects and the associated regulations. Program-specific terms have been defined separately for each program and are contained in pertinent sub-sections of Section 2 of this handbook. ACRONYMS ASTM American Society for Testing Materials CBBEL Christopher B. Burke Engineering, Ltd. COE United States Army Corps of Engineers EPA Environmental Protection Agency IDEM Indiana Department of Environmental Management IDNR Indiana Department of Natural Resources NRCS USDA-Natural Resources Conservation Service SWCD Soil and Water Conservation District USDA United States Department of Agriculture USFWS United States Fish and Wildlife Service DEFINITIONS AASHTO Classification. The official classification of soil materials and soil aggregate mixtures for highway construction used by the American Association of State Highway and Transportation Officials. Abutment. The sloping sides of a valley that supports the ends of a dam. Acre-Foot. The volume of water that will cover 1 acre to a depth of 1 ft. Aggregate. (1) The sand and gravel portion of concrete (65 to 75% by volume), the rest being cement and water. Fine aggregate contains particles ranging from 1/4 in. down to that retained on a 200-mesh screen. Coarse aggregate ranges from 1/4 in. up to l½ in. (2) That which is installed for the purpose of changing drainage characteristics. -

Carbon Dioxide
Safetygram 18 Carbon dioxide Carbon dioxide is nonflammable, colorless, and odorless in the gaseous and liquid states. Carbon dioxide is a minor but important constituent of the atmosphere, averaging about 0.036% or 360 ppm by volume. It is also a normal end-prod- uct of human and animal metabolism. Dry carbon dioxide is a relatively inert gas. In the event moisture is present in high concentrations, carbonic acid may be formed and materials resistant to this acid should be used. High flow rates or rapid depressurization of a system can cause temperatures approaching the sublimation point (–109.3°F [–78.5°C]) to be attained within the system. Carbon dioxide will convert directly from a liquid to a solid if the liquid is depressurized below 76 psia (61 psig). The use of ma- terials which become brittle at low temperatures should be avoided in applications where temperatures less than –20°F (–29°C) are expected. Vessels and piping used in carbon dioxide service should be designed to the American Society of Mechanical Engineers (ASME) or Department of Transportation (DOT) codes for the pressures and temperatures involved. Physical properties are listed in Table 1. Carbon dioxide in the gaseous state is colorless and odorless and not easily detectable. Gaseous carbon dioxide is 1.5 times denser than air and therefore is found in greater concentrations at low levels. Ventilation systems should be designed to exhaust from the lowest levels and allow make-up air to enter at a higher level. Manufacture Carbon dioxide is produced as a crude by-product of a number of manufactur- ing processes. -

A STUDY of the BINARY SYSTEM Na-Cs
C oo|p ef A STUDY OF THE BINARY SYSTEM Na-Cs BY L. M. COOPER THESIS FOR THE DEGREE OF BACHELOR OF SCIENCE IN CHEMICAL ENGINEERING COLLEGE OF LIBERAL ARTS AND SCIENCES UNIVERSITY OF ILLINOIS 1917 1*3 »T UNIVERSITY OF ILLINOIS .Maz..25. i^ilr. THIS IS TO CERTIFY THAT THE THESIS PREPARED UNDER MY SUPERVISION BY LiiOU...M...,..C.O,Qm T3E.. BINARY.. ENTITLED A... SI.UM.. .PI.. S^^^^^ THE IS APPROVED BY ME AS FULFILLING THIS PART OF THE REQUIREMENTS FOR DEGREE OF., BA.GHELOR...Qii...S.C.IM.C.E. Instructor in Charge Approved : HEAD OF DEPARTMENT OF. 3 UHJC -13- IX TABLE OF CONTENTS Page I Introduction 1 II Source and purification of materials 2 III Method 6 IV Table 7 V Temperature-concentration diagram of the binary system Na-Gs 8 VI Discussion of diagram 9 VII Conclusions 11 VIII Bibliography 12 IX Table of Contents 13 X Acknowledgement 14 Digitized by the Internet Archive in 2013 http://archive.org/details/studyofbinarysysOOcoop . I INTRODUCTION Systems of two alkali metals have been little studied. Lithium in its alloy forming properties resembles magnesium rather than sodium, and its behavior is in some respects anom- alous. Molten lithium is hardly miscible with sodium or potas- (1) sium. Sodium forms a single compound NaoK, with potassium, (3) but this compound dissociates below its melting point. It is probable that both lithium and sodium would prove, on investi- gation, to form compounds with rubidium and caesium, but that the metals of the potassium sub-group would not combine with one another In the second sub-group, copper, silver, and gold alloy together, forming solid solutions either completely or to a limited extent. -

Guidelines for the Use of Atomic Weights 5 10 11 12 DOI: ..., Received ...; Accepted
IUPAC Guidelines for the us e of atomic weights For Peer Review Only Journal: Pure and Applied Chemistry Manuscript ID PAC-REC-16-04-01 Manuscript Type: Recommendation Date Submitted by the Author: 01-Apr-2016 Complete List of Authors: van der Veen, Adriaan; VSL Meija, Juris Possolo, Antonio; National Institute of Standards and Technology Hibbert, David; University of New South Wales, School of Chemistry atomic weights, atomic-weight intervals, molecular weight, standard Keywords: atomic weight, measurement uncertainty, uncertainty propagation Author-Supplied Keywords: P.O. 13757, Research Triangle Park, NC (919) 485-8700 Page 1 of 13 IUPAC Pure Appl. Chem. 2016; aop 1 2 3 4 Sponsoring body: IUPAC Inorganic Chemistry Division Committee: see more details on page XXX. 5 IUPAC Recommendation 6 7 Adriaan M. H. van der Veen*, Juris Meija, Antonio Possolo, and D. Brynn Hibbert 8 9 Guidelines for the use of atomic weights 5 10 11 12 DOI: ..., Received ...; accepted ... 13 14 Abstract: Standard atomicFor weights Peer are widely used Review in science, yet the uncertainties Only associated with these 15 values are not well-understood. This recommendation provides guidance on the use of standard atomic 16 weights and their uncertainties. Furthermore, methods are provided for calculating standard uncertainties 17 of molecular weights of substances. Methods are also outlined to compute material-specific atomic weights 10 18 whose associated uncertainty may be smaller than the uncertainty associated with the standard atomic 19 weights. 20 21 Keywords: atomic weights; atomic-weight intervals; molecular weight; standard atomic weight; uncertainty; 22 uncertainty propagation 23 24 25 1 Introduction 15 26 27 Atomic weights provide a practical link the SI base units kilogram and mole. -

Safety Data Sheet According to 1907/2006/EC, Article 31 Printing Date 21.07.2021 Revision: 21.07.2021
Page 1/6 Safety data sheet according to 1907/2006/EC, Article 31 Printing date 21.07.2021 Revision: 21.07.2021 SECTION 1: Identification of the substance/mixture and of the company/undertaking · 1.1 Product identifier · Trade name: Cesium chloride (99.999%-Cs) PURATREM · Item number: 93-5542 · CAS Number: 7647-17-8 · EC number: 231-600-2 · 1.2 Relevant identified uses of the substance or mixture and uses advised against No further relevant information available. · 1.3 Details of the supplier of the safety data sheet · Manufacturer/Supplier: Strem Chemicals, Inc. 7 Mulliken Way NEWBURYPORT, MA 01950 USA [email protected] · Further information obtainable from: Technical Department · 1.4 Emergency telephone number: EMERGENCY: CHEMTREC: + 1 (800) 424-9300 During normal opening times: +1 (978) 499-1600 SECTION 2: Hazards identification · 2.1 Classification of the substance or mixture · Classification according to Regulation (EC) No 1272/2008 The substance is not classified according to the CLP regulation. · 2.2 Label elements · Labelling according to Regulation (EC) No 1272/2008 Void · Hazard pictograms Void · Signal word Void · Hazard statements Void · Precautionary statements P231 Handle under inert gas. P262 Do not get in eyes, on skin, or on clothing. P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P403+P233 Store in a well-ventilated place. Keep container tightly closed. P422 Store contents under inert gas. P501 Dispose of contents/container in accordance with local/regional/national/international regulations. · 2.3 Other hazards · Results of PBT and vPvB assessment · PBT: Not applicable. -

The Water Molecule
Seawater Chemistry: Key Ideas Water is a polar molecule with the remarkable ability to dissolve more substances than any other natural solvent. Salinity is the measure of dissolved inorganic solids in water. The most abundant ions dissolved in seawater are chloride, sodium, sulfate, and magnesium. The ocean is in steady state (approx. equilibrium). Water density is greatly affected by temperature and salinity Light and sound travel differently in water than they do in air. Oxygen and carbon dioxide are the most important dissolved gases. 1 The Water Molecule Water is a polar molecule with a positive and a negative side. 2 1 Water Molecule Asymmetry of a water molecule and distribution of electrons result in a dipole structure with the oxygen end of the molecule negatively charged and the hydrogen end of the molecule positively charged. 3 The Water Molecule Dipole structure of water molecule produces an electrostatic bond (hydrogen bond) between water molecules. Hydrogen bonds form when the positive end of one water molecule bonds to the negative end of another water molecule. 4 2 Figure 4.1 5 The Dissolving Power of Water As solid sodium chloride dissolves, the positive and negative ions are attracted to the positive and negative ends of the polar water molecules. 6 3 Formation of Hydrated Ions Water dissolves salts by surrounding the atoms in the salt crystal and neutralizing the ionic bond holding the atoms together. 7 Important Property of Water: Heat Capacity Amount of heat to raise T of 1 g by 1oC Water has high heat capacity - 1 calorie Rocks and minerals have low HC ~ 0.2 cal. -

Standardless, Automated Determination of Chlorine-35 by 35Cl Nuclear Magnetic Resonance
Standardless, Automated Determination of Chlorine-35 by 35Cl Nuclear Magnetic Resonance Watson, Simon A1; Edwards, Andy J1; Parkinson, John A2 1GlaxoSmithKline Medicines Research Centre, Stevenage, Hertfordshire, UK 2 WestCHEM Department of Pure and Applied Chemistry, University of Strathclyde, Glasgow, UK Corresponding author: Watson, Simon A; GlaxoSmithKline, Gunnelswood Road, Stevenage, Hertfordshire, UK, SG1 2NY; 01438 766685; [email protected] We present an example of a robust, fully automated, walk-up method to quantify chloride concentration in sample solutions using 35Cl nuclear magnetic resonance (NMR). Minimal user input is required, no standards are acquired at the time of analysis and the submission, acquisition, processing and production of results are seamlessly integrated within existing NMR automation software. The method demonstrated good linearity with R2 = 0.999 over three orders of magnitude of analyte concentration, results that are highly independent of analyte functionality, and the stability of instrument response was such that analyses of additional standards were not required for a period of several months. At a nominal sample concentration of 10 mg/ml in D2O at 400 MHz, a detection limit and a quantitation limit of 0.1 and 0.5% w/w, respectively, was achieved in a 1 hour run time. Robust methodology was achieved by applying a rigorous approach to method development and validation to determine and evaluate fully the time- and sample-dependent factors that affect quantitation in this experiment. 1 Keywords: NMR, 35Cl, chloride, quantitation, walk-up, automation, pharmaceutical analysis Introduction The maxim that “nuclear magnetic resonance (NMR) is inherently quantitative” has become a central dogma of NMR spectroscopy in literature and presentations. -
Simple Cubic Lattice
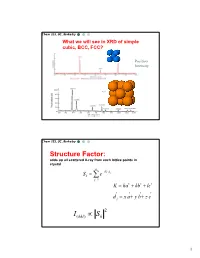
Chem 253, UC, Berkeley What we will see in XRD of simple cubic, BCC, FCC? Position Intensity Chem 253, UC, Berkeley Structure Factor: adds up all scattered X-ray from each lattice points in crystal n iKd j Sk e j1 K ha kb lc d j x a y b z c 2 I(hkl) Sk 1 Chem 253, UC, Berkeley X-ray scattered from each primitive cell interfere constructively when: eiKR 1 2d sin n For n-atom basis: sum up the X-ray scattered from the whole basis Chem 253, UC, Berkeley ' k d k d di R j ' K k k Phase difference: K (di d j ) The amplitude of the two rays differ: eiK(di d j ) 2 Chem 253, UC, Berkeley The amplitude of the rays scattered at d1, d2, d3…. are in the ratios : eiKd j The net ray scattered by the entire cell: n iKd j Sk e j1 2 I(hkl) Sk Chem 253, UC, Berkeley For simple cubic: (0,0,0) iK0 Sk e 1 3 Chem 253, UC, Berkeley For BCC: (0,0,0), (1/2, ½, ½)…. Two point basis 1 2 iK ( x y z ) iKd j iK0 2 Sk e e e j1 1 ei (hk l) 1 (1)hkl S=2, when h+k+l even S=0, when h+k+l odd, systematical absence Chem 253, UC, Berkeley For BCC: (0,0,0), (1/2, ½, ½)…. Two point basis S=2, when h+k+l even S=0, when h+k+l odd, systematical absence (100): destructive (200): constructive 4 Chem 253, UC, Berkeley Observable diffraction peaks h2 k 2 l 2 Ratio SC: 1,2,3,4,5,6,8,9,10,11,12. -

Measuring Density
Measuring Density Background All matter has mass and volume. Mass is a measure of the amount of matter an object has. Its measure is usually given in grams (g) or kilograms (kg). Volume is the amount of space an object occupies. There are numerous units for volume including liters (l), meters cubed (m3), and gallons (gal). Mass and volume are physical properties of matter and may vary with different objects. For example, it is possible for two pieces of metal to be made out of the same material yet for one piece to be bigger than the other. If the first piece of metal is twice as large as the second, then you would expect that this piece is also twice as heavy (or have twice the mass) as the first. If both pieces of metal are made of the same material the ratio of the mass and volume will be the same. We define density (ρ) as the ratio of the mass of an object to the volume it occupies. The equation is given by: M ρ = (1.1) V here the symbol M stands for the mass of the object, and V the volume. Density has the units of mass divided by volume such as grams per centimeters cube (g/cm3) or kilograms per liter (kg/l). Sample Problem #1 A block of wood has a mass of 8 g and occupies a volume of 10 cm3. What is its density? Solution 8g The density will be = 0.8g / cm 3 . 10cm 3 This means that every centimeter cube of this wood will have a mass of 0.8 grams. -

Density Objective Learn How to Calculate Density
Density Objective Learn how to calculate density Density is a way of comparing the mass of an object to its size (volume). If an object feels heavy for its size, it has a high density. A dense object has more material (atoms) packed into a smaller area. Density cannot be measured directly, it must be calculated. The formula used to calculate density is density = mass/volume or D = m/v. The typical label for density is g/mL or g/cm3. Density is a useful tool for scientist. It is often used to help identify a pure substance. A pure substance will have one unique density. For example, each element on the periodic table has a density from any other element. If you had an unknown gas, you could calculate the density of it to identify it. The same is true for minerals- though several minerals may have the same density so other properties would also need to be used to identify the mineral. If you have ever noticed oil floating on water, that is an example of density. Objects that are less dense than water, it will float. Waters density is 1.0g/mL. 1. In general terms, describe what density is. 2. How could density be used to identify an unknown substance? 3. Density must be calculated using a math formula. What is the formula for density? 4. What is a typical label for density? *Obviously both water and ice are the same substance- so should have the same density. But does it? Then why does ice float? Suggest an explanation.