1 a Review on Unitized Regenerative Fuel Cell Technologies, Part B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transformative Renewable Energy Storage Devices Based on Neutral Water Input
Transformative Renewable Energy Storage Devices Based on Neutral Water Input EStStUdtEnergy Storage Systems Update ARPA-E GRIDS Kick-Off 4 November 2010 Team • Proton Energy Systems – DKthADr. Kathy Ayers, PI – Luke Dalton, System Lead – Chris Capuano, Stack Lead – Project Lead; Electrolysis Stack and System; Fuel Cell System • Penn State University – Prof. Mike Hickner – Prof. Chao-Yang Wang – Electrolysis and Fuel Cell Membrane Material; Fuel Cell Stack 2 Proton Energy Systems • Manufacturer of Proton Exchange Membrane (PEM) hydrogen generation products using electrolysis • Founded in 1996 • Headquarters in Wallingford, Connecticut. • ISO 9001:2008 registered • Over 1,200 systems operating in 60 different countries 3 Proton Capabilities and Applications PEM Cell Stacks Complete Systems Storage Solutions • Complete product development, manufacturing & testing • Containerization and hydrogen storage solutions • Integration of electrolysis into RFC systems • Turnkeyyp product installation • World-wide sales and service Power Plants HtTtiHeat Treating SiSemicon dtductors LbLabora tor ies Government 4 HOGEN® C Series 3 • Maximum Capacity: 30 Nm /h H2 (65 kg/day) (~200 kW input) • Commercial availability: Q1 2011 • 5X h y drogen ou tpu t with onl15Xthfly 1.5X the foo t pritint 5 Next Steps in Scale Up • 70 Nm3/h • 150 kg/day • 400 kW input 0.6 SQFT 3 Cell (1032 amps, 425 psi, 50oC) 2.30 2.25 2.20 2.15 2.10 2.05 2.00 1.95 Potential (V) ll 1.90 Cel 1.85 Cell 1 Cell 2 Cell 3 1.80 1.75 0 1000 2000 3000 4000 Run Time (hours) 6 Hydrogen Cost Progression -

NASA Fuel Cell and Hydrogen Activities
NASA Fuel Cell and Hydrogen Activities Presented by: Ian Jakupca Department of Energy Annual Merit Review 30 April 2019 1 Overview • National Aeronautic and Space Administration • Definitions • NASA Near Term Activities • Energy Storage and Power • Batteries • Fuel Cells • Regenerative Fuel Cells • Electrolysis • ISRU • Cryogenics • Review 2 National Aeronautics and Space Administration 3 Acknowledgements NASA has many development activities supported by a number of high quality people across the country. This list only includes the most significant contributors to the development of this presentation. Headquarters • Lee Mason, Space Technology Mission Directorate, Deputy Chief Engineer • Gerald (Jerry) Sanders, Lead for In-Situ Resource Utilization (ISRU) System Capability Leadership Team Jet Propulsion Laboratory • Erik Brandon, Ph.D, Electrochemical Technologies • Ratnakumar Bugga, Ph.D, Electrochemical Technologies Marshall Space Flight Center • Kevin Takada, Environmental Control Systems Kennedy Space Center • Erik Dirschka, PE, Propellant Management Glenn Research Center • William R. Bennett, Photovoltaic and Electrochemical Systems • Fred Elliott, Space Technology Project Office • Ryan Gilligan, Cryogenic and Fluid Systems • Wesley L. Johnson, Cryogenic and Fluid Systems • Lisa Kohout, Photovoltaic and Electrochemical Systems • Dianne Linne, ISRU Project Manager • Phillip J. Smith, Photovoltaic and Electrochemical Systems • Tim Smith, Chief, Space Technology Project Office 4 Electrochemical System Definitions Primary Power Energy Storage Commodity Generation Discharge Power Only Charge + Store + Discharge Chemical Conversion Description Description Description • Energy conversion system that • Stores excess energy for later use • Converts supplied chemical feedstock supplies electricity to customer system • Supplies power when baseline power into useful commodities • Operation limited by initial stored supply (e.g. PV) is no longer available • Requires external energy source (e.g. -

Fuel Cell Performance and Degradation
REVERSIBLE FUEL CELL PERFORMANCE AND DEGRADATION by Matthew Aaron Cornachione A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Electrical Engineering MONTANA STATE UNIVERSITY Bozeman, Montana April, 2011 c Copyright by Matthew Aaron Cornachione 2011 All Rights Reserved ii APPROVAL of a thesis submitted by Matthew Aaron Cornachione This thesis has been read by each member of the thesis committee and has been found to be satisfactory regarding content, English usage, format, citations, bibli- ographic style, and consistency, and is ready for submission to the The Graduate School. Dr. Steven R. Shaw Approved for the Department of Electrical and Computer Engineering Dr. Robert C. Maher Approved for the The Graduate School Dr. Carl A. Fox iii STATEMENT OF PERMISSION TO USE In presenting this thesis in partial fulfullment of the requirements for a master's degree at Montana State University, I agree that the Library shall make it available to borrowers under rules of the Library. If I have indicated my intention to copyright this thesis by including a copyright notice page, copying is allowable only for scholarly purposes, consistent with \fair use" as prescribed in the U.S. Copyright Law. Requests for permission for extended quotation from or reproduction of this thesis in whole or in parts may be granted only by the copyright holder. Matthew Aaron Cornachione April, 2011 iv ACKNOWLEDGEMENTS I would like to thank my advisor, Dr. Steven Shaw, for granting me the oppor- tunity to work at Montana State University as a graduate research assistant and for providing assistance to many aspects of this work from circuit design to machining parts. -

Development of Membraneless Mixed-Reactant Microfluidic Fuel Cells: Electrocatalysis and Evolution Through Numerical Simulation
Université du Québec Institut National de la Recherche Scientifique Centre Énergie, Matériaux et Télécommunications DEVELOPMENT OF MEMBRANELESS MIXED-REACTANT MICROFLUIDIC FUEL CELLS: ELECTROCATALYSIS AND EVOLUTION THROUGH NUMERICAL SIMULATION Par Juan Carlos Abrego Martínez Thèse présentée pour l’obtention du grade de Philosophiae Doctor (Ph.D.) en sciences de l’énergie et des matériaux Jury d’évaluation Président du jury et Andreas Ruediger examinateur interne Professeur à l’INRS-ÉMT Examinateur externe Ricardo Izquierdo Professeur à l’École de technologie supérieure (ETS) Examinateur externe Sasha Omanovic Professeur à l’Université de McGill Directeur de recherche Mohamed Mohamedi Professeur à l’INRS-ÉMT Codirecteur de recherche Shuhui Sun Professeur à l’INRS-ÉMT © Droits réservés de Juan Carlos Abrego Martínez, 4 Juin 2020 ACKNOWLEDGMENTS First and foremost, I would like to thank my supervisor, Prof. Mohamed Mohamedi for offering me the opportunity to undertake my PhD studies in his research group, for providing the tools and trusting me to carry out this project and for the excellent guidance and support throughout this period. I also express my gratitude to my co-supervisor, Prof. Shuhui Sun for closely following the research progress and for his valuable contribution for accomplishing my PhD project. I thank the Jury members, Prof. Andreas Ruediger, Prof. Ricardo Izquierdo and Prof. Sasha Omanovic for agreeing and taking the time to review and evaluate this work. I would like to thank my group colleagues, Youling Wang, Alonso Moreno, Haixia Wang, Naser Mohammadi, Xiaoying Zheng, Khawtar Hasan and Soraya Bouachma, for sharing their knowledge and experience through discussions and experiments in the laboratory. -

Co-Laminar Flow Cells for Electrochemical Energy Conversion
Co-laminar flow cells for electrochemical energy conversion by Marc-Antoni Goulet M.Sc. (Physics), McMaster University, 2008 B.Sc., McGill University, 2006 Dissertation Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in the School of Engineering Science Faculty of Applied Sciences Marc-Antoni Goulet 2016 SIMON FRASER UNIVERSITY Summer 2016 Approval Name: Marc-Antoni Goulet Degree: Doctor of Philosophy Title: Co-laminar flow cells for electrochemical energy conversion Examining Committee: Chair: Amr Marzouk Lecturer Erik Kjeang Senior Supervisor Associate Professor Michael Eikerling Supervisor Professor Gary Wang Supervisor Professor Edward Park Internal Examiner Professor Matthew Mench External Examiner Professor Department of Mechanical, Aerospace and Biomedical Engineering University of Tennessee, Knoxville Date Defended/Approved: May 2nd, 2016 ii Abstract A recently developed class of electrochemical cell based on co-laminar flow of reactants through porous electrodes is investigated. New architectures are designed and assessed for fuel recirculation and rechargeable battery operation. Extensive characterization of cells is performed to determine most sources of voltage loss during operation. To this end, a specialized flow cell technique is developed to mitigate mass transport limitations and measure kinetic rates of reaction on flow-through porous electrodes. This technique is used in in conjunction with cyclic voltammetry and electrochemical impedance spectroscopy to evaluate different treatments for enhancing the rates of vanadium redox reactions on carbon paper electrodes. It is determined that surface area enhancements are the most effective way for increasing redox reaction rates and thus a novel in situ flowing deposition method is conceived to achieve this objective at minimal cost. -

1 a Switchable Ph-Differential Unitized Regenerative Fuel Cell with High
A switchable pH-differential unitized regenerative fuel cell with high performance Xu Lu,a Jin Xuan,be Dennis Y.C. Leung,*a Haiyang Zou,a Jiantao Li,ac Hailiang Wang d and Huizhi Wang *b a Department of Mechanical Engineering, The University of Hong Kong, Pok Fu Lam, Hong Kong b Institute of Mechanical, Process and Energy Engineering, School of Engineering and Physical Sciences, Heriot-Watt University, Edinburgh, EH14 4AS, UK c SINOPEC Fushun Research Institute of Petroleum and Petrochemicals, Fushun, China d Department of Chemistry, Yale University, West Haven, CT, United States e State-Key Laboratory of Chemical Engineering, School of Mechanical and Power Engineering, East China University of Science and Technology, Shanghai 200237, China Correspondence and requests for materials should be addressed to D.Y.C.L. (email: [email protected]) or to H.Z.W. (email: [email protected]). 1 Abstract Regenerative fuel cells are a potential candidate for future energy storage, but their applications are limited by the high cost and poor round-trip efficiency. Here we present a switchable pH- differential unitized regenerative fuel cell capable of addressing both the obstacles. Relying on a membraneless laminar flow-based design, pH environments in the cell are optimized independently for different electrode reactions and are switchable together with the cell process to ensure always favorable thermodynamics for each electrode reaction. Benefiting from the thermodynamic advantages of the switchable pH-differential arrangement, the cell allows water electrolysis at a voltage of 0.57 V, and a fuel cell open circuit voltage of 1.89 V, rendering round-trip efficiencies up to 74%. -
DOE Hydrogen Program 2010 Annual Merit Review and Peer Evaluation
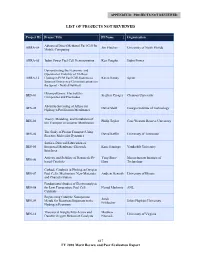
APPENDIX D: PROJECTS NOT REVIEWED LIST OF PROJECTS NOT REVIEWED Project ID Project Title PI Name Organization Advanced Direct Methanol Fuel Cell for ARRA-04 Jim Fletcher University of North Florida Mobile Computing ARRA-05 Jadoo Power Fuel Cell Demonstration Ken Vaughn Jadoo Power Demonstrating the Economic and Operational Viability of 72-Hour ARRA-12 Hydrogen PEM Fuel Cell Systems to Kevin Kenny Sprint Support Emergency Communications on the Sprint - Nextel Network Fluoropolymers, Electrolytes, BES-01 Stephen Creager Clemson University Composites and Electrodes Ab-initio Screening of Alloys for BES-02 David Sholl Georgia Institute of Technology Hydrogen Purification Membranes Theory, Modeling, and Simulation of BES-03 Philip Taylor Case Western Reserve University Ion Transport in Ionomer Membranes The Study of Proton Transport Using BES-04 David Keffer University of Tennessee Reactive Molecular Dynamics Surface-Directed Fabrication of BES-05 Integrated Membrane-Electrode Kane Jennings Vanderbilt University Interfaces Activity and Stability of Nanoscale Pt- Yang Shao- Massachusetts Institute of BES-06 based Catalysts Horn Technology Cathode Catalysis in Hydrogen/Oxygen BES-07 Fuel Cells: Mechanism, New Materials, Andrew Gewirth University of Illinois and Characterization Fundamental Studies of Electrocatalysis BES-08 for Low Temperature Fuel Cell Nenad Markovic ANL Catalysts Engineering Catalytic Nanoporous Jonah BES-09 Metals for Reactions Important to the Johns Hopkins University Erlebacher Hydrogen Economy Theoretical Insights Into -

Membraneless Hydrogen Bromine Laminar Flow Battery for Large
Membraneless Hydrogen Bromine Laminar Flow Battery for Large-Scale Energy Storage by William Allan Braff Submitted to the Department of Mechanical Engineering in partial fulfillment of the requirements for the degree of Doctor of Philosophy at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY February 2014 c Massachusetts Institute of Technology 2014. All rights reserved. Author.............................................................. Department of Mechanical Engineering December 19, 2013 Certified by. Cullen R. Buie Assistant Professor of Mechanical Engineering Thesis Supervisor Certified by. Martin Z. Bazant Professor of Chemical Engineering and Mathematics Thesis Supervisor Accepted by . David E. Hardt Chairman, Department Committee on Graduate Theses 2 Membraneless Hydrogen Bromine Laminar Flow Battery for Large-Scale Energy Storage by William Allan Braff Submitted to the Department of Mechanical Engineering on December 19, 2013, in partial fulfillment of the requirements for the degree of Doctor of Philosophy Abstract Electrochemical energy storage systems have been considered for a range of potential large-scale energy storage applications. These applications vary widely, both in the order of magnitude of energy storage that is required and the rate at which energy must be charged and discharged. One such application aids the integration of renew- able energy technologies onto the electrical grid by shifting the output from renewable energy resources to periods of high demand, relaxing transmission and distribution requirements and reducing the need for fossil fuel burning plants. Although the mar- ket need for such solutions is well known, existing technologies are still too expensive to compete with conventional combustion-based solutions. In this thesis, the hydrogen bromine laminar flow battery (HBFLB) is proposed and examined for its potential to provide low cost energy storage using the rapid reaction kinetics of hydrogen-bromine reaction pairs and a membrane-less laminar flow battery architecture. -

Relating Catalysis Between Fuel Cell and Metal-Air Batteries
Perspective Relating Catalysis between Fuel Cell and Metal-Air Batteries Matthew Li,1,2 Xuanxuan Bi,1 Rongyue Wang,3 Yingbo Li,4,6 Gaopeng Jiang,2 Liang Li,5 Cheng Zhong,6,* Zhongwei Chen,2,* and Jun Lu1,* With the ever-increasing demand for higher-performing energy-storage sys- Progress and Potential tems, electrocatalysis has become a major topic of interest in an attempt to Catalyst research for fuel cells has enhance the electrochemical performance of many electrochemical technolo- led to much advancement in gies. Discoveries pertaining to the oxygen reduction reaction catalyst helped humanity’s understanding of the enable the commercialization of fuel-cell-based electric vehicles. However, a underlying physics of the process, closely related technology, the metal-air battery, has yet to find commercial significantly enhancing the application. Much like the Li-ion battery, metal-air batteries can potentially uti- performance of the technologies. lize the electrical grid network for charging, bypassing the need for establishing In contrast, metal-air batteries a hydrogen infrastructure. Among the metal-air batteries, Li-air and Zn-air bat- such as Li-air and Zn-air batteries teries have drawn much interest in the past decade. Unfortunately, state-of-the remain to be solved. Although the art metal-air batteries still produce performances that are well below practical metal anode used in this these levels. In this brief perspective, we hope to bridge some of the ideas from systems does play a large role in fuel cell to that of metal-air batteries with the aim of inspiring new ideas and di- limiting their commercial success, rections for future research. -

Bifunctional Oxygen Reduction/Evolution Catalysts for Rechargeable Metal-Air
Bifunctional Oxygen Reduction/Evolution Catalysts for Rechargeable Metal-Air Batteries and Regenerative Alkaline Fuel Cells by Pooya Hosseini-Benhangi M.Sc., Ferdowsi University of Mashhad, 2011 B.Sc., Ferdowsi University of Mashhad, 2009 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in THE FACULTY OF GRADUATE AND POSTDOCTORAL STUDIES (Chemical and Biological Engineering) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) December 2016 © Pooya Hosseini-Benhangi, 2016 Abstract The electrocatalysis of oxygen reduction and evolution reactions (ORR and OER, respectively) on the same catalyst surface is among the long-standing challenges in electrochemistry with paramount significance for a variety of electrochemical systems including regenerative fuel cells and rechargeable metal-air batteries. Non-precious group metals (non- PGMs) and their oxides, such as manganese oxides, are the alternative cost-effective solutions for the next generation of high-performance bifunctional oxygen catalyst materials. Here, initial stage electrocatalytic activity and long-term durability of four non-PGM oxides and their combinations, i.e. MnO2, perovskites (LaCoO3 and LaNiO3) and fluorite-type oxide (Nd3IrO7), were investigated for ORR and OER in alkaline media. The combination of structurally diverse oxides revealed synergistic catalytic effect by improved bifunctional activity compared to the individual oxide components. Next, the novel role of alkali-metal ion insertion and the mechanism involved for performance promotion of oxide catalysts were investigated. Potassium insertion in the oxide structures enhanced both ORR and OER performances, e.g. 110 and 75 mV decrease in the OER (5 mAcm-2) -2 and ORR (-2 mAcm ) overpotentials (in absolute values) of MnO2-LaCoO3, respectively, during galvanostatic polarization tests. -

Solar-PV & Fuel Cell Based Hybrid Power Solution for Remote Locations
International Journal of Engineering and Advanced Technology (IJEAT) ISSN: 2249 – 8958, Volume-9 Issue-1, October 2019 Solar-PV & Fuel Cell Based Hybrid Power Solution for Remote Locations Manish Kumar Singla, Parag Nijhawan, Amandeep Singh Oberoi Abstract: Inherently variable nature of renewable sources of Currently available batteries in market are lithium-based energy such as solar and wind, are incapable of meeting which are heavy, toxic, and expensive to recycle. One continuous supply demand. Combining solar photovoltaic (PV) solution to all such problems is generation of hydrogen from and fuel cell could offer a feasible solution to the challenge of renewable energy source (e.g. solar PVs), its storage and continuous power supply, particularly in those geographical reuse in a fuel cell to give back electricity when renewable locations where renewable resources are available in abundance. The present paper investigates a solar PV and fuel cell-based sources are not available. To investigate the feasibility of hybrid system in-context to a selected site in Indian sub- such a hybrid system a theoretical study is conducted for a continent. The feasibility of harnessing renewable energy per sq. selected site – Jodhpur located in north of India and meter of land (i.e. energy density) from a combined solar PV-fuel simulation on solar irradiance is carried out in PVsyst cell based hybrid system employed in Jodhpur location in software. The fixed input parameters of the selected site are Rajasthan is reported. The solar irradiance data for the last three inserted to obtain the results in terms of power generation decades corresponding to the longitudinal and latitudinal and its variance through-out the year. -

Development of an Efficient Future Energy Storage System Incorporating Fluidized Bed Of
DEVELOPMENT OF AN EFFICIENT FUTURE ENERGY STORAGE SYSTEM INCORPORATING FLUIDIZED BED OF MICRO-PARTICLES By Ibitoye Adebowale Adelusi Student ID: 31435902 Supervisors Dr Dawson and Dr Andrieux Dissertation for the degree of Doctor of Philosophy Lancaster University Engineering Department 06/06/2019 1 | P a g e Declaration This research work: numerical modeling and simulation and laboratory experiments and write- up of this thesis DEVELOPMENT OF AN EFFICIENT FUTURE ENERGY STORAGE SYSTEM INCORPORATING FLUIDIZED BED OF MICRO-PARTICLES was carried out by me. I also confirm that the Lancaster university laboratory equipment’s and numerical software’s such as solidwork, ANSYS and COMSOL have been used to acquire all the results. In terms of the referencing, I have used Lancaster University library one search tools to access all the resources such as scientific journals that were related to the research work of this thesis. Furthermore, I can confirm that: before or after submitting this dissertation, the following papers that are listed below will be published. 1. Practical Development of a ZnBr2 Flow Battery with a Fluidized Bed Anode Zinc- Electrode Journal of The Electrochemical Society, Volume 167, Number 5 Focus Issue, 2019 – Published. 2. Hybrid Zinc Based Redox Flow Batteries as a renewable energy source: an overview – Pending. 3. Simulation of Added Carbon Particles Within Fluidized Bed Anode Zinc-Electrode – Pending Additionally, my supervisors, Dr Andrieux and Dr Dawson have checked every written word of this dissertation, supervised me during the research work and corrected and checked all my experimental work acquired results (numerical modelling and laboratory) and recommended to me most of the conferences and training that I had attended during this research work and close to completion.