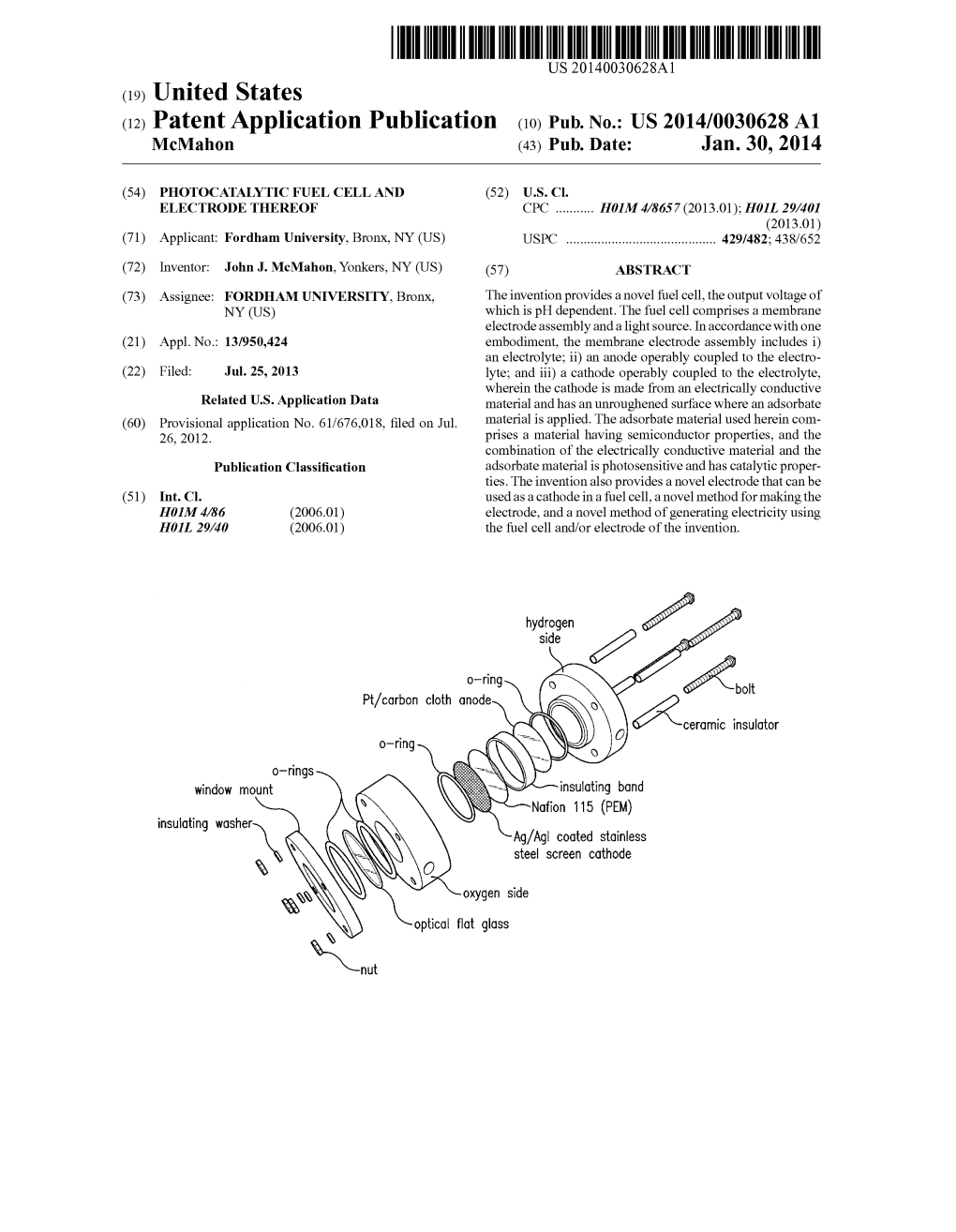
S). 6. Insulator N) Insulating Band Nafion 115 (PEM)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Bergen Phd Thesis
Integration and Dynamics of a Renewable Regenerative Hydrogen Fuel Cell System by Alvin Peter Bergen B.A.Sc., University of Victoria, 1994 M.A.Sc., University of Victoria, 1999 A Dissertation Submitted in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY in the Department of Mechanical Engineering © Alvin Bergen, 2008 University of Victoria All rights reserved. This thesis may not be reproduced in whole or in part, by photocopy or other means, without the permission of the author. ISBN: 978-0-494-41177-3 ii Supervisory Committee Integration and Dynamics of a Renewable Regenerative Hydrogen Fuel Cell System by Alvin Peter Bergen B.A.Sc., University of Victoria, 1994 M.Sc., University of University, 1999 Supervisory Committee Dr. Ned Djilali, Department of Mechanical Engineering Supervisor Dr. Peter Wild, Department of Mechanical Engineering Supervisor Dr. Andrew Rowe, Department of Mechanical Engineering Departmental Member Dr. Tom Fyles, Department of Chemistry Outside Member Dr. Brant Peppley, Department of Chemical Engineering, Queen’s University External Examiner iii Abstract Supervisory Committee Dr. Ned Djilali, Department of Mechanical Engineering Supervisor Dr. Peter Wild, Department of Mechanical Engineering Supervisor Dr. Andrew Rowe, Department of Mechanical Engineering Departmental Member Dr. Tom Fyles, Department of Chemistry Outside Member Dr. Brant Peppley, Department of Chemical Engineering, Queen’s University External Examiner This thesis explores the integration and dynamics of residential scale renewable- regenerative energy systems which employ hydrogen for energy buffering. The development of the Integrated Renewable Energy Experiment (IRENE) test-bed is presented. IRENE is a laboratory-scale distributed energy system with a modular structure which can be readily re-configured to test newly developed components for generic regenerative systems. -

Transformative Renewable Energy Storage Devices Based on Neutral Water Input
Transformative Renewable Energy Storage Devices Based on Neutral Water Input EStStUdtEnergy Storage Systems Update ARPA-E GRIDS Kick-Off 4 November 2010 Team • Proton Energy Systems – DKthADr. Kathy Ayers, PI – Luke Dalton, System Lead – Chris Capuano, Stack Lead – Project Lead; Electrolysis Stack and System; Fuel Cell System • Penn State University – Prof. Mike Hickner – Prof. Chao-Yang Wang – Electrolysis and Fuel Cell Membrane Material; Fuel Cell Stack 2 Proton Energy Systems • Manufacturer of Proton Exchange Membrane (PEM) hydrogen generation products using electrolysis • Founded in 1996 • Headquarters in Wallingford, Connecticut. • ISO 9001:2008 registered • Over 1,200 systems operating in 60 different countries 3 Proton Capabilities and Applications PEM Cell Stacks Complete Systems Storage Solutions • Complete product development, manufacturing & testing • Containerization and hydrogen storage solutions • Integration of electrolysis into RFC systems • Turnkeyyp product installation • World-wide sales and service Power Plants HtTtiHeat Treating SiSemicon dtductors LbLabora tor ies Government 4 HOGEN® C Series 3 • Maximum Capacity: 30 Nm /h H2 (65 kg/day) (~200 kW input) • Commercial availability: Q1 2011 • 5X h y drogen ou tpu t with onl15Xthfly 1.5X the foo t pritint 5 Next Steps in Scale Up • 70 Nm3/h • 150 kg/day • 400 kW input 0.6 SQFT 3 Cell (1032 amps, 425 psi, 50oC) 2.30 2.25 2.20 2.15 2.10 2.05 2.00 1.95 Potential (V) ll 1.90 Cel 1.85 Cell 1 Cell 2 Cell 3 1.80 1.75 0 1000 2000 3000 4000 Run Time (hours) 6 Hydrogen Cost Progression -

NASA Fuel Cell and Hydrogen Activities
NASA Fuel Cell and Hydrogen Activities Presented by: Ian Jakupca Department of Energy Annual Merit Review 30 April 2019 1 Overview • National Aeronautic and Space Administration • Definitions • NASA Near Term Activities • Energy Storage and Power • Batteries • Fuel Cells • Regenerative Fuel Cells • Electrolysis • ISRU • Cryogenics • Review 2 National Aeronautics and Space Administration 3 Acknowledgements NASA has many development activities supported by a number of high quality people across the country. This list only includes the most significant contributors to the development of this presentation. Headquarters • Lee Mason, Space Technology Mission Directorate, Deputy Chief Engineer • Gerald (Jerry) Sanders, Lead for In-Situ Resource Utilization (ISRU) System Capability Leadership Team Jet Propulsion Laboratory • Erik Brandon, Ph.D, Electrochemical Technologies • Ratnakumar Bugga, Ph.D, Electrochemical Technologies Marshall Space Flight Center • Kevin Takada, Environmental Control Systems Kennedy Space Center • Erik Dirschka, PE, Propellant Management Glenn Research Center • William R. Bennett, Photovoltaic and Electrochemical Systems • Fred Elliott, Space Technology Project Office • Ryan Gilligan, Cryogenic and Fluid Systems • Wesley L. Johnson, Cryogenic and Fluid Systems • Lisa Kohout, Photovoltaic and Electrochemical Systems • Dianne Linne, ISRU Project Manager • Phillip J. Smith, Photovoltaic and Electrochemical Systems • Tim Smith, Chief, Space Technology Project Office 4 Electrochemical System Definitions Primary Power Energy Storage Commodity Generation Discharge Power Only Charge + Store + Discharge Chemical Conversion Description Description Description • Energy conversion system that • Stores excess energy for later use • Converts supplied chemical feedstock supplies electricity to customer system • Supplies power when baseline power into useful commodities • Operation limited by initial stored supply (e.g. PV) is no longer available • Requires external energy source (e.g. -

Fuel Cell Performance and Degradation
REVERSIBLE FUEL CELL PERFORMANCE AND DEGRADATION by Matthew Aaron Cornachione A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Electrical Engineering MONTANA STATE UNIVERSITY Bozeman, Montana April, 2011 c Copyright by Matthew Aaron Cornachione 2011 All Rights Reserved ii APPROVAL of a thesis submitted by Matthew Aaron Cornachione This thesis has been read by each member of the thesis committee and has been found to be satisfactory regarding content, English usage, format, citations, bibli- ographic style, and consistency, and is ready for submission to the The Graduate School. Dr. Steven R. Shaw Approved for the Department of Electrical and Computer Engineering Dr. Robert C. Maher Approved for the The Graduate School Dr. Carl A. Fox iii STATEMENT OF PERMISSION TO USE In presenting this thesis in partial fulfullment of the requirements for a master's degree at Montana State University, I agree that the Library shall make it available to borrowers under rules of the Library. If I have indicated my intention to copyright this thesis by including a copyright notice page, copying is allowable only for scholarly purposes, consistent with \fair use" as prescribed in the U.S. Copyright Law. Requests for permission for extended quotation from or reproduction of this thesis in whole or in parts may be granted only by the copyright holder. Matthew Aaron Cornachione April, 2011 iv ACKNOWLEDGEMENTS I would like to thank my advisor, Dr. Steven Shaw, for granting me the oppor- tunity to work at Montana State University as a graduate research assistant and for providing assistance to many aspects of this work from circuit design to machining parts. -

Hydrogen Fuel Cell Vehicle and a Stand- Alone Renewable Energy-Based Refuelling Station
HYDROGEN FUEL CELL VEHICLE AND A STAND- ALONE RENEWABLE ENERGY-BASED REFUELLING STATION Master’s Thesis University of Jyväskylä Department of Biological and Environmental Science Master’s Degree Programme in Renewable Energy Atte Pakkanen 20th August, 2007 2 JYVÄSKYLÄ UNIVERSITY - Faculty of Mathematics and Science - Department of Biological and Environmental Science - Master’s Degree Programme in Renewable Energy Pakkanen Atte: Hydrogen fuel cell vehicle and a stand-alone renewable energy-based refuelling station Master´s Thesis: 107 p., 2 appendixes (2 p.) Supervisors: Dr. Mohanlal Kolhe and D.Sc. (Tech.) Margareta Wihersaari Inspectors: Dr. Mohanlal Kolhe and Dr. Jussi Maunuksela August 2007 Key words: hydrogen, fuel cell, hydrogen vehicle, hydrogen storage, metal hydride storage, electrolysis, renewable energy, alternative transportation fuels ABSTRACT Energy use for transportation purposes and the number of passenger vehicles is increasing rapidly around the world. Fuelling infrastructure based on hydrogen provides an attractive alternative to predominating fossil fuels. This thesis project includes research on hydrogen fuel cell vehicle and its local refuelling station based on hydrogen production from renewable energy sources. Hydrogen produced via electrolysis is stored in portable metal hydride containers to be used in a fuel cell hybrid vehicle. The test vehicle is equipped with a battery unit and two 6 kW electric motors. The targets of this thesis project were to test and estimate the driving range of the vehicle, to examine the behaviour of the metal hydride containers under changing temperatures and determine the rate of hydrogen production via electrolysis. Also hydrogen leak tests to assure safe testing procedures in the future were included to these initial tests. -

1 a Switchable Ph-Differential Unitized Regenerative Fuel Cell with High
A switchable pH-differential unitized regenerative fuel cell with high performance Xu Lu,a Jin Xuan,be Dennis Y.C. Leung,*a Haiyang Zou,a Jiantao Li,ac Hailiang Wang d and Huizhi Wang *b a Department of Mechanical Engineering, The University of Hong Kong, Pok Fu Lam, Hong Kong b Institute of Mechanical, Process and Energy Engineering, School of Engineering and Physical Sciences, Heriot-Watt University, Edinburgh, EH14 4AS, UK c SINOPEC Fushun Research Institute of Petroleum and Petrochemicals, Fushun, China d Department of Chemistry, Yale University, West Haven, CT, United States e State-Key Laboratory of Chemical Engineering, School of Mechanical and Power Engineering, East China University of Science and Technology, Shanghai 200237, China Correspondence and requests for materials should be addressed to D.Y.C.L. (email: [email protected]) or to H.Z.W. (email: [email protected]). 1 Abstract Regenerative fuel cells are a potential candidate for future energy storage, but their applications are limited by the high cost and poor round-trip efficiency. Here we present a switchable pH- differential unitized regenerative fuel cell capable of addressing both the obstacles. Relying on a membraneless laminar flow-based design, pH environments in the cell are optimized independently for different electrode reactions and are switchable together with the cell process to ensure always favorable thermodynamics for each electrode reaction. Benefiting from the thermodynamic advantages of the switchable pH-differential arrangement, the cell allows water electrolysis at a voltage of 0.57 V, and a fuel cell open circuit voltage of 1.89 V, rendering round-trip efficiencies up to 74%. -

Metal Hydride Hydrogen Storage and Compression Systems for Energy Storage Technologies
international journal of hydrogen energy 46 (2021) 13647e13657 Available online at www.sciencedirect.com ScienceDirect journal homepage: www.elsevier.com/locate/he Metal hydride hydrogen storage and compression systems for energy storage technologies Boris P. Tarasov a,*, Pavel V. Fursikov a, Alexey A. Volodin a, Mikhail S. Bocharnikov a, Yustinas Ya Shimkus a, Aleksey M. Kashin b, Volodymyr A. Yartys c, Stanford Chidziva d, Sivakumar Pasupathi d, Mykhaylo V. Lototskyy d,** a Institute of Problems of Chemical Physics (IPCP) of Russian Academy of Sciences, Chernogolovka, 142432, Russia b InEnergy Group, Moscow, 115201, Russia c Institute for Energy Technology, Kjeller NO, 2027, Norway d HySA Systems Competence Centre, South African Institute for Advanced Materials Chemistry (SAIAMC), University of the Western Cape, Bellville, 7535, South Africa highlights Use of metal hydride storage and compression in hydrogen energy storage systems. AB5- and AB2-type hydrides for hydrogen storage and compression applications. Development of the energy storage systems and their metal hydride based components. article info abstract Article history: Along with a brief overview of literature data on energy storage technologies utilising Received 29 February 2020 hydrogen and metal hydrides, this article presents results of the related R&D activities Received in revised form carried out by the authors. The focus is put on proper selection of metal hydride materials 6 July 2020 on the basis of AB5- and AB2-type intermetallic compounds for hydrogen storage and Accepted 9 July 2020 compression applications, based on the analysis of PCT properties of the materials in Available online 6 August 2020 systems with H2 gas. The article also presents features of integrated energy storage sys- tems utilising metal hydride hydrogen storage and compression, as well as their metal Keywords: hydride based components developed at IPCP and HySA Systems. -

Relating Catalysis Between Fuel Cell and Metal-Air Batteries
Perspective Relating Catalysis between Fuel Cell and Metal-Air Batteries Matthew Li,1,2 Xuanxuan Bi,1 Rongyue Wang,3 Yingbo Li,4,6 Gaopeng Jiang,2 Liang Li,5 Cheng Zhong,6,* Zhongwei Chen,2,* and Jun Lu1,* With the ever-increasing demand for higher-performing energy-storage sys- Progress and Potential tems, electrocatalysis has become a major topic of interest in an attempt to Catalyst research for fuel cells has enhance the electrochemical performance of many electrochemical technolo- led to much advancement in gies. Discoveries pertaining to the oxygen reduction reaction catalyst helped humanity’s understanding of the enable the commercialization of fuel-cell-based electric vehicles. However, a underlying physics of the process, closely related technology, the metal-air battery, has yet to find commercial significantly enhancing the application. Much like the Li-ion battery, metal-air batteries can potentially uti- performance of the technologies. lize the electrical grid network for charging, bypassing the need for establishing In contrast, metal-air batteries a hydrogen infrastructure. Among the metal-air batteries, Li-air and Zn-air bat- such as Li-air and Zn-air batteries teries have drawn much interest in the past decade. Unfortunately, state-of-the remain to be solved. Although the art metal-air batteries still produce performances that are well below practical metal anode used in this these levels. In this brief perspective, we hope to bridge some of the ideas from systems does play a large role in fuel cell to that of metal-air batteries with the aim of inspiring new ideas and di- limiting their commercial success, rections for future research. -

Innovative Research and Products, Inc. En-104
iiRRAAPP DIRECTORY AND COMPANY PROFILES - Fuel Cells, Hydrogen Energy and Related Nanotechnology INNOVATIVE RESEARCH AND PRODUCTS, INC. EN-104 DIRECTORY AND COMPANY PROFILES - FUEL CELLS, HYDROGEN ENERGY AND RELATED NANOTECHNOLOGY Alton Parish Project Analyst INNOVATIVE RESEARCH AND PRODUCTS ( iRAP ), INC. P.O. Box 16760 Stamford, CT 06905-8760 Tel: (203) 569-7909 Web: www.innoresearch.net Email: [email protected] Copyright2009@Innovative Research and Products, Inc., Stamford, CT U.S., Web: www.innoresearch.net DIRECTORY AND COMPANY PROFILES - Fuel Cells, Hydrogen Energy and Related Nanotechnology ABOUT iRAP Innovative Research and Products ( iRAP ), Inc. conducts market research and industry analysis in new generation technologies and products. Areas covered include advanced materials, nanotechnology and nonmaterial processing technologies and products, advanced ceramics, metals and alloys, high- performance coatings, automotive components, aircraft and aerospace materials, electronic devices, photonic components, membranes, plastics, pharmaceutical products and biotechnology. CUSTOM ANALYSIS iRAP will be happy to talk to you about your market research needs if you need to expand on the current market survey to cover a new product or technology. We are confident that we will meet your expectations and provide you the most accurate industry and market analysis. Send us an email outlining your market research and industry analysis objectives and we will provide you a FREE quote. TERMS OF PURCHASE By purchasing this report, the purchaser is bound to the following conditions: • The report can only be used by the group or the division which bought this report. • The report cannot be copied and sent to other groups or divisions in the same company or other companies. -

Bifunctional Oxygen Reduction/Evolution Catalysts for Rechargeable Metal-Air
Bifunctional Oxygen Reduction/Evolution Catalysts for Rechargeable Metal-Air Batteries and Regenerative Alkaline Fuel Cells by Pooya Hosseini-Benhangi M.Sc., Ferdowsi University of Mashhad, 2011 B.Sc., Ferdowsi University of Mashhad, 2009 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in THE FACULTY OF GRADUATE AND POSTDOCTORAL STUDIES (Chemical and Biological Engineering) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) December 2016 © Pooya Hosseini-Benhangi, 2016 Abstract The electrocatalysis of oxygen reduction and evolution reactions (ORR and OER, respectively) on the same catalyst surface is among the long-standing challenges in electrochemistry with paramount significance for a variety of electrochemical systems including regenerative fuel cells and rechargeable metal-air batteries. Non-precious group metals (non- PGMs) and their oxides, such as manganese oxides, are the alternative cost-effective solutions for the next generation of high-performance bifunctional oxygen catalyst materials. Here, initial stage electrocatalytic activity and long-term durability of four non-PGM oxides and their combinations, i.e. MnO2, perovskites (LaCoO3 and LaNiO3) and fluorite-type oxide (Nd3IrO7), were investigated for ORR and OER in alkaline media. The combination of structurally diverse oxides revealed synergistic catalytic effect by improved bifunctional activity compared to the individual oxide components. Next, the novel role of alkali-metal ion insertion and the mechanism involved for performance promotion of oxide catalysts were investigated. Potassium insertion in the oxide structures enhanced both ORR and OER performances, e.g. 110 and 75 mV decrease in the OER (5 mAcm-2) -2 and ORR (-2 mAcm ) overpotentials (in absolute values) of MnO2-LaCoO3, respectively, during galvanostatic polarization tests. -

Solar-PV & Fuel Cell Based Hybrid Power Solution for Remote Locations
International Journal of Engineering and Advanced Technology (IJEAT) ISSN: 2249 – 8958, Volume-9 Issue-1, October 2019 Solar-PV & Fuel Cell Based Hybrid Power Solution for Remote Locations Manish Kumar Singla, Parag Nijhawan, Amandeep Singh Oberoi Abstract: Inherently variable nature of renewable sources of Currently available batteries in market are lithium-based energy such as solar and wind, are incapable of meeting which are heavy, toxic, and expensive to recycle. One continuous supply demand. Combining solar photovoltaic (PV) solution to all such problems is generation of hydrogen from and fuel cell could offer a feasible solution to the challenge of renewable energy source (e.g. solar PVs), its storage and continuous power supply, particularly in those geographical reuse in a fuel cell to give back electricity when renewable locations where renewable resources are available in abundance. The present paper investigates a solar PV and fuel cell-based sources are not available. To investigate the feasibility of hybrid system in-context to a selected site in Indian sub- such a hybrid system a theoretical study is conducted for a continent. The feasibility of harnessing renewable energy per sq. selected site – Jodhpur located in north of India and meter of land (i.e. energy density) from a combined solar PV-fuel simulation on solar irradiance is carried out in PVsyst cell based hybrid system employed in Jodhpur location in software. The fixed input parameters of the selected site are Rajasthan is reported. The solar irradiance data for the last three inserted to obtain the results in terms of power generation decades corresponding to the longitudinal and latitudinal and its variance through-out the year. -

Fundamental Studies of Electrochemical Reactions and Microfluidics in Proton Exchange Membrane Electrolyzer Cells
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2016 Fundamental Studies of Electrochemical Reactions and Microfluidics in Proton Exchange Membrane Electrolyzer Cells Jingke Mo University of Tennessee, Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Energy Systems Commons, and the Propulsion and Power Commons Recommended Citation Mo, Jingke, "Fundamental Studies of Electrochemical Reactions and Microfluidics in Proton Exchange Membrane Electrolyzer Cells. " PhD diss., University of Tennessee, 2016. https://trace.tennessee.edu/utk_graddiss/4151 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Jingke Mo entitled "Fundamental Studies of Electrochemical Reactions and Microfluidics in Proton Exchange Membrane Electrolyzer Cells." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Aerospace Engineering. Feng-Yuan Zhang, Major Professor We have read this dissertation and recommend its acceptance: Matthew M. Mench, Zhili Zhang, Lloyd M. Davis Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) Fundamental Studies of Electrochemical Reactions and Microfluidics in Proton Exchange Membrane Electrolyzer Cells A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville Jingke Mo December 2016 Copyright © 2016 by Jingke Mo All rights reserved.