Download Article (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New York Non-Native Plant Invasiveness Ranking Form
NEW YORK NON-NATIVE PLANT INVASIVENESS RANKING FORM Scientific name: Digitalis lanata Ehrh. USDA Plants Code: DILA3 Common names: Grecian foxglove Native distribution: Southeastern Europe Date assessed: February 1, 2010 Assessors: Steve Glenn, Gerry Moore Reviewers: LIISMA SRC Date Approved: March 10, 2010 Form version date: 10 July 2009 New York Invasiveness Rank: Insignificant (Relative Maximum Score <40.00) Distribution and Invasiveness Rank (Obtain from PRISM invasiveness ranking form) PRISM Status of this species in each PRISM: Current Distribution Invasiveness Rank 1 Adirondack Park Invasive Program Not Assessed Not Assessed 2 Capital/Mohawk Not Assessed Not Assessed 3 Catskill Regional Invasive Species Partnership Not Assessed Not Assessed 4 Finger Lakes Not Assessed Not Assessed 5 Long Island Invasive Species Management Area Not Present Low 6 Lower Hudson Not Assessed Not Assessed 7 Saint Lawrence/Eastern Lake Ontario Not Assessed Not Assessed 8 Western New York Not Assessed Not Assessed Invasiveness Ranking Summary Total (Total Answered*) Total (see details under appropriate sub-section) Possible 1 Ecological impact 40 (30) 3 2 Biological characteristic and dispersal ability 25 (22) 13 3 Ecological amplitude and distribution 25 (25) 13 4 Difficulty of control 10 (10) 3 b a Outcome score 100 (87) 32 † Relative maximum score 36.78 § New York Invasiveness Rank Insignificant (Relative Maximum Score <40.00) * For questions answered “unknown” do not include point value in “Total Answered Points Possible.” If “Total Answered Points Possible” is less than 70.00 points, then the overall invasive rank should be listed as “Unknown.” †Calculated as 100(a/b) to two decimal places. §Very High >80.00; High 70.00−80.00; Moderate 50.00−69.99; Low 40.00−49.99; Insignificant <40.00 Not Assessable: not persistent in NY, or not found outside of cultivation. -

Systematics of Gratiola (Plantaginaceae)
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 5-2008 Systematics of Gratiola (Plantaginaceae) Larry D. Estes University of Tennessee - Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Ecology and Evolutionary Biology Commons Recommended Citation Estes, Larry D., "Systematics of Gratiola (Plantaginaceae). " PhD diss., University of Tennessee, 2008. https://trace.tennessee.edu/utk_graddiss/381 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Larry D. Estes entitled "Systematics of Gratiola (Plantaginaceae)." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Ecology and Evolutionary Biology. Randall L. Small, Major Professor We have read this dissertation and recommend its acceptance: Edward E. Schilling, Karen W. Hughes, Sally P. Horn Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) To the Graduate Council: I am submitting herewith a dissertation written by Larry Dwayne Estes entitled “Systematics of Gratiola (Plantaginaceae).” I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Doctor of Philosophy, with a major in Ecology and Evolution. -

The Foxgloves (Digitalis) Revisited*
Reviews The Foxgloves (Digitalis) Revisited* Author Wolfgang Kreis Affiliation Supporting information available online at Lehrstuhl Pharmazeutische Biologie, Department Biology, http://www.thieme-connect.de/products FAU Erlangen-Nürnberg, Erlangen, Germany ABSTRACT Key words Digitalis, Plantaginaceae, cardiac glycosides, plant biotech- This review provides a renewed look at the genus Digitalis. nology, biosynthesis, plant tissue culture, phylogeny Emphasis will be put on those issues that attracted the most attention or even went through paradigmatic changes since received March 17, 2017 the turn of the millennium. PubMed and Google Scholar were “ ” “ ” revised April 27, 2017 used ( Digitalis and Foxglove were the key words) to iden- accepted May 8, 2017 tify research from 2000 till 2017 containing data relevant enough to be presented here. Intriguing new results emerged Bibliography from studies related to the phylogeny and taxonomy of the DOI https://doi.org/10.1055/s-0043-111240 genus as well as to the biosynthesis and potential medicinal Published online May 23, 2017 | Planta Med 2017; 83: 962– uses of the key active compounds, the cardiac glycosides. 976 © Georg Thieme Verlag KG Stuttgart · New York | Several Eastern and Western Foxgloves were studied with re- ISSN 0032‑0943 spect to their propagation in vitro. In this context, molecular biology tools were applied and phytochemical analyses were Correspondence conducted. Structure elucidation and analytical methods, Prof. Dr. Wolfgang Kreis which have experienced less exciting progress, will not be Department Biology, FAU Erlangen-Nürnberg considered here in great detail. Staudtstr. 5, 91058 Erlangen, Germany Phone:+4991318528241,Fax:+4991318528243 [email protected] Taxus species is a prime example [4]. -

Botanist Interior 43.1
2005 THE MICHIGAN BOTANIST 81 NOTEWORTHY COLLECTIONS: MINNESOTA AND WISCONSIN David J. Schimpf and Deborah L. Pomroy Olga Lakela Herbarium Department of Biology University of Minnesota Duluth, MN 55812-3003 [email protected] Rumex stenophyllus Ledeb. (Polygonaceae). Narrowleaf Dock. Previous knowledge: Rumex stenophyllus is a herbaceous perennial native to moist, often saline, soils from central Europe to central Asia (Löve & Bernard 1958). Its known naturalized North American range (Löve & Bernard 1958, USDA 2004) comes nearest to Wisconsin in the Twin Cities area of Minnesota (Ownbey & Morley 1991). Significance. Two populations of R. stenophyllus were found in Superior, Wisconsin, apparently the first known for the state. Both included numerous in- dividuals in tall weedy herbaceous vegetation on upland clay soil. A soil test of the Catlin site found the electrical conductivity to be 0.3 dS/m, which is a non- saline value (Lal 2002). 1 WISCONSIN. DOUGLAS CO.: W of Catlin Ave., at ca. 2400 block, Superior, SE ⁄4 Sec. 23, T49N R14W, 27 Jul 2003, Schimpf 343 (DUL, SUWS, WIS); former petroleum tank farm, 1 Superior, SE ⁄4 Sec. 16, T49N R14W, 27 Jul 2003, Schimpf 344 (WIS). Acer platanoides L. (Aceraceae). Norway Maple. Previous knowledge. Acer platanoides is a shade-tolerant, deciduous tree na- tive to Europe and cultivated in North America (Gleason & Cronquist 1991). It is escaped or naturalized in states east of Minnesota or in the Pacific Northwest (USDA 2004), as well as in eastern Canada (Scoggan 1978). Although shown as occurring outside of cultivation in Minnesota by USDA (2004), wild Minnesota specimens of A. -

Botanist Interior 38.3
38 THE MICHIGAN BOTANIST Vol. 38 NOTEWORTHY COLLECTIONS MINNESOTA DIGITALIS GRANDIFLORA Miller (Scrophulariaceae). Yellow Foxglove. Previous knowledge. Digitalis grandiflora is a biennial or perennial native to Eurasia, where it grows in woods (Tutin et al. 1972). In eastern North America, D. grandiflora occasionally escapes from cultivation (Magee & Ahles 1999). D. grandiflora has been reported outside of cultivation from Baraga and Houghton Counties in upper Michigan (Voss 1996). We are not aware of other collections from the upper Great Lakes. Significance. A population of D. grandiflora was found in Duluth, Minnesota, apparently the first escape of this species in the state. The plants were on a steep, open northeast-facing bank about 1 km from Lake Superior. This is a residential neighborhood, in which the population’s founders may have been cultivated. The species was absent from a similar bank across the avenue, suggesting that spread by seed is weak. The plants had large rhizomes bearing numerous scars from flowering stems, and have been observed flowering vigorously every year since their discovery. We infer that D. grandiflora behaves as a perennial in this envi- ronment, allowing it to persist and spread locally even if reproduction by seed is ineffective. This species contains some of the same cardiac glycosides as D. lanata Ehrh. and D. purpurea L. (Hollman 1985), suggesting that caution be taken to prevent skin contact or ingestion by humans, livestock, or pets. MINNESOTA. ST. LOUIS CO.: narrow patch about 12 m long between sidewalk and over- grown lot, plants in flower, with Campanula rapunculoides L., SW side of 24th Ave. -

Research on Spontaneous and Subspontaneous Flora of Botanical Garden "Vasile Fati" Jibou
Volume 19(2), 176- 189, 2015 JOURNAL of Horticulture, Forestry and Biotechnology www.journal-hfb.usab-tm.ro Research on spontaneous and subspontaneous flora of Botanical Garden "Vasile Fati" Jibou Szatmari P-M*.1,, Căprar M. 1 1) Biological Research Center, Botanical Garden “Vasile Fati” Jibou, Wesselényi Miklós Street, No. 16, 455200 Jibou, Romania; *Corresponding author. Email: [email protected] Abstract The research presented in this paper had the purpose of Key words inventory and knowledge of spontaneous and subspontaneous plant species of Botanical Garden "Vasile Fati" Jibou, Salaj, Romania. Following systematic Jibou Botanical Garden, investigations undertaken in the botanical garden a large number of spontaneous flora, spontaneous taxons were found from the Romanian flora (650 species of adventive and vascular plants and 20 species of moss). Also were inventoried 38 species of subspontaneous plants, adventive plants, permanently established in Romania and 176 vascular plant floristic analysis, Romania species that have migrated from culture and multiply by themselves throughout the garden. In the garden greenhouses were found 183 subspontaneous species and weeds, both from the Romanian flora as well as tropical plants introduced by accident. Thus the total number of wild species rises to 1055, a large number compared to the occupied area. Some rare spontaneous plants and endemic to the Romanian flora (Galium abaujense, Cephalaria radiata, Crocus banaticus) were found. Cultivated species that once migrated from culture, accommodated to environmental conditions and conquered new territories; standing out is the Cyrtomium falcatum fern, once escaped from the greenhouses it continues to develop on their outer walls. Jibou Botanical Garden is the second largest exotic species can adapt and breed further without any botanical garden in Romania, after "Anastasie Fătu" care [11]. -

Ornamental and Garden Plants: Controlling Deer Damage
Oklahoma Cooperative Extension Service HLA-6427 Ornamental and Garden Plants: Controlling Deer Damage David Hillock Extension Consumer Horticulturist Oklahoma Cooperative Extension Fact Sheets are also available on our website at: Kimberly Toscano http://osufacts.okstate.edu Extension Consumer Horticulturist Dwayne Elmore Extension Wildlife Specialist The first step in managing deer damage in the land- scape is to make the landscape less attractive to deer. This Oklahoma’s white-tailed deer (Odocoileus virginianus) is accomplished by limiting the amount of excess food in (Figure 1) population has increased from 40,000 to around the landscape through removing all unharvested fruits and 500,000 since the 1960s. At the same time, urban development vegetables. Do not provide winter feed or salt for deer as an continues to move into deer habitat. Increasingly, homeown- alternative to your landscape plants; the deer will feed on both ers at the rural/urban interface must deal with deer damage the deer feed and your plants. When deer damage becomes to ornamental and garden plants. As deer begin moving into a problem in the landscape, control methods include: an area, homeowners initially enjoy seeing deer and may 1) exclusion—by electric fence or eight-foot high, deer-proof actually encourage them to come into their yard by feeding fence, them. Homeowner attitudes often begin to change after deer 2) scare or frightening tactics—with dogs, gas exploders, numbers increase to the extent that landscape plants show fireworks or motion-activated sprinklers, heavy browsing and gardens become difficult to grow because 3) population reduction through hunting, of continued depredation. -
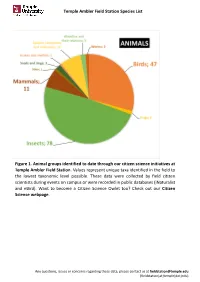
Temple Ambler Field Station Species List Figure 1. Animal Groups Identified to Date Through Our Citizen Science Initiatives at T
Temple Ambler Field Station Species List Figure 1. Animal groups identified to date through our citizen science initiatives at Temple Ambler Field Station. Values represent unique taxa identified in the field to the lowest taxonomic level possible. These data were collected by field citizen scientists during events on campus or were recorded in public databases (iNaturalist and eBird). Want to become a Citizen Science Owlet too? Check out our Citizen Science webpage. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station Species List Figure 2. Plant diversity identified to date in the natural environments and designed gardens of the Temple Ambler Field Station and Ambler Arboretum. These values represent unique taxa identified to the lowest taxonomic level possible. Highlighted are 14 of the 116 flowering plant families present that include 524 taxonomic groups. A full list can be found in our species database. Cultivated specimens in our Greenhouse were not included here. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station Species List database_title Temple Ambler Field Station Species List last_update 22October2020 description This database includes all species identified to their lowest taxonomic level possible in the natural environments and designed gardens on the Temple Ambler campus. These are occurrence records and each taxon is only entered once. This is an occurrence record, not an abundance record. IDs were performed by senior scientists and specialists, as well as citizen scientists visiting campus. -

Digitalis Grandiflora Mill
Common Names: › Foxglove, Big-Flowered Foxglove Family Scrophulariaceae – Figwort family Genus Digitalis L. – foxglove Species Digitalis grandiflora Mill.– yellow foxglove Scientific Name › Digitalis grandiflora http://plants.usda.gov/java/profile? symbol=DIGR4 Eastern Europe › Digitalis purpurea The United States › Minneosta › Maine › Ohio › Michigan › Several Northeastern States (grandiflora) Ontario, CA Latitude Ranges › 3-85 Altitude › Sea level to 8,000 ft Site Conditions Plant Traits Special Considerations Sunlight Life Cycle -Deer resistant -Partial shade -Perennial -Non aggressive Soil conditions Ease of Care But will self-seed under -Requires well- -Easy favorable conditions drained media Height -Non-native -High fertility -1.5 to 3 feet -not native to North Spread -1 to 1.5 feet America - Native to *Tolerates a wide Bloom-time central and southern range of soil -Early summer Europe, Siberia and conditions, except for -Mid-summer Asia Minor very wet or very dry. Flower color -All parts of the flower Performs best in rich -Yellow are poisonous soil that is moist but Foliage Color -Dark green well-drained. U.S. Hardiness Zone Shape Special Uses -Zone 3 to 8 -cushion, mound or -Cut flower clump This species thrives in acidic soils in a range of habitats including open woods, woodland clearings, on moorland and heath margins, hedge banks, sea-cliffs, waste land, rocky mountain slopes and hedgebanks. It is common in disturbed sites, or on burnt ground Clump forming perennial Native to woods and stream banks in Central -

WUCOLS List S Abelia Chinensis Chinese Abelia M ? ? M / / Copyright © UC Regents, Davis Campus
Ba Bu G Gc P Pm S Su T V N Botanical Name Common Name 1 2 3 4 5 6 Symbol Vegetation Used in Type WUCOLS List S Abelia chinensis Chinese abelia M ? ? M / / Copyright © UC Regents, Davis campus. All rights reserved. bamboo Ba S Abelia floribunda Mexican abelia M ? M M / / S Abelia mosanensis 'Fragrant Abelia' fragrant abelia ? ? ? ? ? ? bulb Bu S Abelia parvifolia (A. longituba) Schuman abelia ? ? ? M ? ? grass G groundcover GC Gc S Abelia x grandiflora and cvs. glossy abelia M M M M M / perennial* P S Abeliophyllum distichum forsythia M M ? ? ? ? palm and cycad Pm S Abelmoschus manihot (Hibiscus manihot) sunset muskmallow ? ? ? L ? ? T Abies pinsapo Spanish fir L L L / / / shrub S succulent Su T N Abies spp. (CA native and non-native) fir M M M M / / P N Abronia latifolia yellow sand verbena VL VL VL / ? ? tree T P N Abronia maritima sand verbena VL VL VL / ? ? vine V California N native S N Abutilon palmeri Indian mallow L L L L M M S Abutilon pictum thompsonii variegated Chinese lantern M H M M ? ? Sunset WUCOLS CIMIS ET Representative Number climate 0 Region zones** Cities zones* S Abutilon vitifolium flowering maple M M M / ? ? Healdsburg, Napa, North- San Jose, Salinas, Central 14, 15, 16, 17 1, 2, 3, 4, 6, 8 San Francisco, Coastal San Luis Obispo S Abutilon x hybridum & cvs. flowering maple M H M M / / 1 Auburn, Central Bakersfield, Chico, 8, 9, 14 12, 14, 15, 16 Valley Fresno, Modesto, Sacramento S T Acacia abyssinica Abyssinian acacia / ? / ? / L 2 Irvine, Los South Angeles, Santa 22, 23, 24 1, 2, 4, 6 Coastal Barbara, Ventura, -

Southern Ontario Vascular Plant Species List
Southern Ontario Vascular Plant Species List (Sorted by Scientific Name) Based on the Ontario Plant List (Newmaster et al. 1998) David J. Bradley Southern Science & Information Section Ontario Ministry of Natural Resources Peterborough, Ontario Revised Edition, 2007 Southern Ontario Vascular Plant Species List This species checklist has been compiled in order to assist field biologists who are sampling vegetative plots in Southern Ontario. It is not intended to be a complete species list for the region. The intended range for this vascular plant list is Ecoregions (Site Regions) 5E, 6E and 7E. i Nomenclature The nomenclature used for this listing of 2,532 plant species, subspecies and varieties, is in accordance with the Ontario Plant List (OPL), 1998 [see Further Reading for full citation]. This is the Ontario Ministry of Natural Resource’s publication which has been selected as the corporate standard for plant nomenclature. There have been many nomenclatural innovations in the past several years since the publication of the Ontario Plant List that are not reflected in this listing. However, the OPL has a listing of many of the synonyms that have been used recently in the botanical literature. For a more up to date listing of scientific plant names visit either of the following web sites: Flora of North America - http://www.efloras.org/flora_page.aspx?flora_id=1 NatureServe - http://www.natureserve.org/explorer/servlet/NatureServe?init=Species People who are familiar with the Natural Heritage Information Centre (NHIC) plant species list for Ontario, will notice some changes in the nomenclature. For example, most of the Aster species have now been put into the genus Symphyotrichum, with a few into the genus Eurybia. -

European Red List of Medicinal Plants
European Red List of Medicinal Plants Compiled by David Allen, Melanie Bilz, Danna J. Leaman, Rebecca M. Miller, Anastasiya Timoshyna and Jemma Window European Red List of Medicinal Plants Compiled by David Allen, Melanie Bilz, Danna J. Leaman, Rebecca M. Miller, Anastasiya Timoshyna and Jemma Window IUCN Global Species Programme IUCN European Union Representative Office IUCN Species Survival Commission Published by the European Commission. The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN or the European Union concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN or the European Union. Citation: Allen, D., Bilz, M., Leaman, D.J., Miller, R.M., Timoshyna, A. and Window, J. 2014. European Red List of Medicinal Plants. Luxembourg: Publications Office of the European Union. Design and layout: Imre Sebestyén jr. / UNITgraphics.com Printed by: Rosseels Printing Picture credits on cover page: Artemisia granatensis is endemic to the mountains of Sierra Nevada, southern Spain. The plant is considered Endangered as a result of population decline and range contraction. ©José Quiles Hoyo / www.florasilvestre.es All photographs used in this publication remain the property of the original copyright holder (see individual captions for details). Photographs should