Life Sciences and Healthcare
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Full Report
CONTENTS 02 Unstoppable Corporate overview We have been persistent 02 Unstoppable in our aim to establishing 04 Chairman’s Message and maintaining market leadership to be able to achieve 06 Vice Chairman’s Message unprecedented growth for our 08 Board of Directors stakeholders. 10 Management Board 12 Key Performance Highlights 14 14 Integrated Report Integrated Report 28 Management Discussion & Analysis Apollo Tyres’ contribution 51 Sustainability Snapshot to social and economic development is critical to create and sustain an enabling environment for investment. This has enabled the Company’s positioning as a credible Statutory Reports stakeholder partner. 84 Board’s Report 95 Annual Report on CSR 28 102 Business Responsibility Report Management 126 Corporate Governance Report Discussion & Analysis Financial Statements 161 Standalone Financial Statements 223 Consolidated Financial Statements UNSTOPPABLE Since inception, we have worked towards establishing ourselves as a leading player in the sale and manufacture of tyres. We have been persistent in our aim to establishing and maintaining market leadership and be able to achieve unprecedented growth for our stakeholders. In FY2018-19 (FY2019), we continued to focus We are unstoppable in establishing our on our key revenue generating regions APMEA leadership across multiple segments. (Asia Pacific, Middle East and Africa, including Our consistently advancing product range coupled India) and Europe. We expanded our presence in with product innovations are enabling us to achieve Americas by added new territories and increasing the same. our value proposition. The APMEA operation continued its focus on consolidating its leadership We are unstoppable in working towards achieving and enhancing share in India through the cutting edge manufacturing capabilities and introduction of best in class and technologically world-class R&D. -

Consumer Goods Recovery in Discretionary and Urban Sales Led to Better Q3 Sector Update
Consumer Goods Recovery in discretionary and urban sales led to better Q3 Sector Update Consumer goods companies’ Q3 performance was driven by sales recovery of Q3FY2021 Results Review discretionary categories (such as value-added hair oil and personal care products), sustained higher demand for healthcare and hygiene products, better traction to Sector: Consumer Goods new launches, and higher demand in rural markets coupled with improving demand in urban markets. General trade continues to grow strongly, e-commerce mix to Sector View: Positive overall revenue is improving due to higher sales and modern trade channel has witnessed sequential improvement due to recovery in urban sales. Most consumer goods companies under our coverage registered organic revenue growth of 6%-16%, driven by domestic volume growth of 7%-18% in Q3. Paint companies, including Asian Paints, registered strong volume growth of 30%, led by sustained high demand in tier III/IV towns and improving demand in metros and top cities due to receding scare of virus and improving construction and real estate activities. Overall, Sharekhan’s consumer goods universe registered revenue growth of ~14% in Q3FY2021, better than 9.1% growth achieved in Q2FY2021. Significant increase Our coverage universe in prices of palm oil, copra, other edible oils, and raw tea/coffee resulted in gross Companies CMP Reco. PT margin decline for companies such as HUL, Godrej Consumer Products (GCPL), (Rs) (Rs) Marico, and Tata Consumer Products (TCPL). However, lower ad spends and cost- Asian Paints 2,389 Buy 3,000 saving initiatives arrested the sharp decline of 80-100 bps in operating profit margins (OPM) for some companies. -

Emami Companyname
RESULT UPDATE EMAMI Health and rural focus to bear fruit India Equity Research| Consumer Goods COMPANYNAME Emami’s consolidated Q1FY21 revenue (down 25.8% YoY), EBITDA (down EDELWEISS 4D RATINGS 8.3% YoY) and PAT (up 1.2% YoY) were better than our estimates. The Absolute Rating BUY lockdown affected performance in April and May, but June (domestic Rating Relative to Sector Performer business up 8% YoY) turned out to be well. The recovery has sustained Risk Rating Relative to Sector High with double-digit growth in July. Soft mentha prices expanded gross Sector Relative to Market Underweight margin 231bps YoY; with mentha prices remaining benign, we expect gross margin expansion to sustain. A sharp cut in ad spends (down 749bps YoY) led to 487bps YoY EBITDA margin expansion. The lockdown MARKET DATA (R: EMAM.BO, B: HMN IN) affected international business (IB) as well overall sales dipped 18% YoY CMP : INR 257 Target Price : INR 296 (however up 7% YoY in June). Going ahead, with promoter-level pledging 52-week range (INR) : 358 / 131 concern alleviating in the wake of the recent Emami Cement sale, a slew Share in issue (mn) : 444.5 of launches in health & hygiene to capitalise on the topical upswing, and M cap (INR bn/USD mn) : 115 / 1,510 a higher rural contribution should hold the company in good stead. Avg. Daily Vol.BSE/NSE(‘000) : 958.1 Maintain ‘BUY’ with a TP of INR296. SHARE HOLDING PATTERN (%) Health & hygiene portfolio gains; discretionary portfolio suffers Current Q4FY20 Q3FY20 Key highlights: i) 43% of portfolio, which is aligned towards health & hygiene, grew Promoters * 53.9 52.7 52.7 29% YoY while the balance fell 44% YoY. -

IN the BUSINESS of PROGRESS CONTENTS Apollo Tyres in Brief
Annual Report 2019-20 IN THE BUSINESS OF PROGRESS CONTENTS Apollo Tyres in brief 01-03 Apollo Tyres is one of the most trusted Corporate factsheet names in the manufacture and sale of tyres. The Company was founded in 1972 and is 04-13 headquartered in Gurugram, Haryana (India). Leadership and governance 06 Chairman’s message 08 Vice-Chairman’s message 10 Board of Directors Catering to all tyre segments 12 Management Team TRUCK AND BUS LIGHT TRUCK 14-77 Performance and progress 16 Key performance indicators 18 Know our capitals 20 Business model 22 Capital-wise information 36 Progress amidst volatility 38 Progress that is sustainable 40 Progress through people centricity 42 Value created for the stakeholders PASSENGER VEHICLES TWO-WHEELER 78-93 Management Discussion & Analysis 94-170 Statutory Reports 94 Board’s Report 107 Annual Report on CSR 113 Business Responsibility Report 138 Corporate Governance Report OFF-HIGHWAY 171-305 Financial Statements 171 Standalone 233 Consolidated Apollo Tyres’ success as a leading tyre such as: geo-political relations, economic manufacturer is inextricably linked with the growth, industry cyclicality, environmental progress of the people, the businesses we issues, technological innovation, safety and partner and the planet at large. We have consumer attitudes. Our FY20 performance is always persevered to exceed expectations, a reflection of the resulting uncertainties. It set new benchmarks and in some cases, is also a testament to our foundational values shape the future of the industry. and intrinsic strengths that have helped us navigate through these uncertainties. Our marquee brands, Apollo and Vredestein, enjoy premium positions in the commercial As we step into a new decade, replete with and passenger vehicle tyre segments in unexplored opportunities, we are keen on India and Europe, respectively. -

HSBC Saudi Arabia Limited
HSBC Saudi Arabia Limited HSBC China & India Equity Freestyle Fund - IAF Fund Details Monthly Factsheet Fund Manager HSBC Saudi Arabia Ltd. as of 31 January 2015 Inception Date 12 December 2005 Profile Inception Price USD 10 To provide capital appreciation through investing in a well-diversified portfolio comprising of shares in Indian and Chinese companies over a long period of five years and above . Fund Type Open ended Top Holdings Investment Policy Freestyle Management Stock Weight % Base Currency US Dollar of the Fund Tencent Holdings Ltd. 10 Baidu Inc. Sponsored ADR 9 Risk/Return Profile High Infosys Ltd. 8 Bloomberg Code SABCHIN AB Wipro Ltd. 7 Emami Ltd. 5 Zawya Code HSBCIEF.MF Oil & Natural Gas Corp. Ltd. 4 Fund Size (USD) 44 Million HCL Technologies Ltd. 4 Minimum Initial USD 2,000 Maruti Suzuki India Ltd. 4 Investment Cognizant Tech Solutions 4 Glenmark Pharmaceuticals Ltd. 4 Minimum Additional USD 1,000 Investment Fund Composition Valuation Day Monday & Thursday Cut off Time Before close of business; On Sunday for Monday valuation, and on Wednesday for Thursday valuation. Redeemed Funds Four business days Payment after valuation day Annual Management Fee 2% Subscription Fee Up to 2% Contact Details HSBC Saudi Arabia Limited Local Investors Toll Free Number 800 124 1212 International Investors Tel +966 1 299 2313 / +966 1 299 2314 Website www.hsbcsaudi.com Email: [email protected] Issued by HSBC Saudi Arabia Limited Authorized and regulated by Capital Market Authority License No. 05008-37 Please note that the above figures refer to past performance and that past performance is not a reliable indicator of future results. -
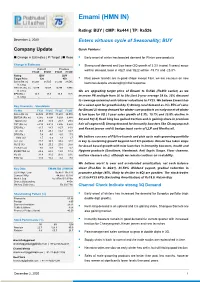
Emami (HMN IN)
Emami (HMN IN) Rating: BUY | CMP: Rs444 | TP: Rs526 December 2, 2020 Enters virtuous cycle of Seasonality; BUY Company Update Quick Pointers: Change in Estimates | ☑ Target | Reco . Early onset of winter has boosted demand for Winter care products Change in Estimates . Strong rural demand and Low base (3Q growth of 3.3% in past 5 years) augur Current Previous well for demand, base in 4Q21 and 1Q22 will be -19.7% and -25.8% FY22E FY23E FY22E FY23E Rating BUY BUY Target Price 526 450 . Most power brands are in good shape except F&H, we are cautious on new Sales (Rs. m) 31,293 34,585 31,293 34,585 launches despite encouraging initial response % Chng. - - EBITDA (Rs. m) 9,299 9,988 9,299 9,988 % Chng. - - We are upgrading target price of Emami to Rs526 (Rs450 earlier) as we EPS (Rs.) 16.7 18.8 16.7 18.8 % Chng. - - increase PE multiple from 26 to 28x (last 5-year average 38.6x, 25% discount to coverage universe) and rollover valuations to FY23. We believe Emami has Key Financials - Standalone hit a sweet spot for growth led by 1) Strong rural demand as it is 55% of sales Y/e Mar FY20 FY21E FY22E FY23E for Emami 2) strong demand for winter care products on early onset of winter Sales (Rs. m) 26,549 27,780 31,293 34,585 3) low base for 3Q ( 5 year sales growth of 3.3%, 19.7% and 25.8% decline in EBITDA (Rs. m) 6,905 8,594 9,299 9,988 4Q and 1Q) 4) Kesh king has gained traction and is gaining share in premium Margin (%) 26.0 30.9 29.7 28.9 PAT (Rs. -

Oman Chapter) an Open Access Journal DOI: 10.12816/0044909 Special Issue: PECTEAM 2018
Arabian J Bus Manag Review (Oman Chapter) An Open Access Journal DOI: 10.12816/0044909 Special Issue: PECTEAM 2018 Arabian Journal of Business and Management Review (Oman Chapter) ResearchResearch Article Homepage: www.arabianjbmr.com AGJ A STUDY ON APPLICATION OF BRAND VALUATION TECHNIQUES WITH REFERENCE TO FAST MOVING CONSUMER GOODS COMPANIES Aisha Banu Research Scholar - Bangalore University Presidency College, Kempapura, Hebbal, Bangalore – 560 024 Dr. Lily David Research Supervisor, Bangalore University St.Joseph College of Commerce, Bangalore Dr. K R Pundareekavittala Co-Research Supervisor Presidency College, Kempapura, Hebbal, Bangalore -560024 Abstract Brand Valuation for the purpose of technical valuation results In balance sheet reporting, tax planning, mergers and acquisitions and investor relations purpose. Though there are various brand valuation techniques available, none can be applied in order to arrive at more accurate value of brand. In light of this drawback, this research paper has been taken up to derive an appropriate brand valuation technique or model suitable to Fast Moving Consumer Goods Companies. The researcher has gathered top ten companies from FMCG based on market capitalisation. Their four years annual reports are analysed, using statistical techniques to substantiate the model arrived at. Introduction Brand refers to any name, design, symbol or any other feature that differentiates one product from another. It is intangible which includes trade names, trademarks, trade symbols, domain names, design rights, trade dress, packaging, copyrights, associated goodwill and advertising visuals. Brand is an intangible asset and very important to a wide variety of industries. The relative importance of intangible asset like brand varies industry wise. For a Pharmaceutical industry, knowledge asset is important, in a Retailing industry Business process asset is significant, for an Airline industry market position asset is primary driver and thus goes the list for various industries. -

Annexure to the Board Report | L&T Annual Report 2019-20
Annexure ‘A’ to the Board Report and EnPI reduction of CG moulding energy consumption. Information as required to be given under Section 134(3) zz Implemented Smart COMM Energy Management (m) read with Rule 8(3) of the Companies (Accounts) system at ASW & Digital Dashboard. Rules, 2014. zz Replacement of conventional light fittings with [A] CONSERVATION OF ENERGY: Solar lighting system in SSII, Open yard-5 and (i) Steps taken or impact on conservation of Grit blasting & painting areas at Production/ energy: Utility areas at EWL Kancheepuram factory and Kansbahal works. zz Implementation of LED lights in HE-Hazira campus and other project sites and Solar Pipes in zz Replacement of conventional MH Lamps and SG fabrication area. fluorescent tube lights by LED lamps in working areas at office and projects as well as for street zz Installation of an Off Grid Mini-Solar Power Plant for meeting the energy requirement of site & lights. workmen habitats at Ranchi Smart City Project. zz Installation of energy efficient water coolers and submersible pumps zz Installed Local Pre/ Post Weld Heat Treatment (PWHT) using PID Technology which ensures zz Replacing existing aged inefficient Split AC units uniform heating and reduction in energy with energy efficient units wastage. zz Utilization of Chiller for HVAC System – Campus zz Implemented the use of Metal Halide (400 Watt) FMD initiated and control the chiller running EOT Crane under bay lights with LED Lights. hour for HVAC need during holidays and extended working hours. zz Installed Energy efficient burners for Furnaces and pre heating. zz Initiative has been taken for replacement of Air-Cooled Chiller with Water Cooled Chiller. -

20130118104321Small366.Pdf
Contents PERFORMANCE REPORT 2006-7 Forward looking statement In this Annual Report we have disclosed forward-looking information to enable investors to comprehend our prospects and take informed investment decisions. This report and other statements that we 10 periodically make contain forward-looking Yesterday’s statements that set out anticipated results wisdom, based on the management’s plans and tomorrow’s assumptions. We have tried wherever products possible to identify such statements by using words such as ‘anticipate’, Why does a nation of 1.1 billion ‘estimate’, ‘expects’, ‘projects’, ‘intends’, Cover story 30 consumers trust Emami? ‘plans’, ‘believes’, and words of similar substance in connection with any India’s FMCG success Wealth at discussion of future performance. story and Emami 18 the bottom of 26 Readers should bear in mind that we the pyramid cannot guarantee that these forward- looking statements will be realised, although we believe we have been prudent in our assumptions. The achievement of 12 results is subject to risks, uncertainties and The fame game: why brands estimates taken as assumptions. Should need celebrities known or unknown risks or uncertainties materialise, or should underlying Enhancing returns, Indian realty sector: assumptions prove inaccurate, actual 40 creating wealth 42 Gaining visibility results could vary materially from those anticipated, estimated or projected. 28 Emami and its global footprint 05 Inauguration of the new corporate office Also Opinions. Appreciation 02 Emami as a Group 46 -

Registered Msos As on 03.08.2020 S.No
Registered MSOs as on 03.08.2020 S.No. Name of MSO Address for Correspondence State Type of Entity Registration No. Date of issue Registation Phone No. Email Remarks of Valid Registration Upto (DD/M M/YYYY) 1 5 Star Network Surpura Road, Bahel Haryana Proprietorship 9/240/2016-DAS 31-10-2016 30-10-2026 98122 45678 5starnetworkbehal@gmail. Bhiwani – 127028 com 2 9 Star Digital Cable D.No. 15-195, Karampudi Road, Andhra Pradesh Partnership 9/109/2015-DAS 24-06-2016 23-06-2026 98483 18777 Palnadu.communications@ Network Gurazala gmail.com Dist. Guntur – 522415 3 A B C O Plot No.6, Ashok Nagar , Odisha Partnership 9/97/2016-DAS 17-05-2016 16-05-2026 98614 44555 [email protected] Bhubaneswar Opp. State Bank of Hyderabad, District Khurda – 751009 4 A Boss Digital System Murugandha Bhavanam, Tamil Nadu Proprietorship 9/491/2015-DAS 17-05-2016 16-05-2026 98421 66931 [email protected] 14-C AA Road Madurai – 625016 5 A– Vision Channel Vrindavan Colony Chhattisgarh Proprietorship 9/77/2016-DAS 26-02-2016 25-02-2026 94252 58909 [email protected] Jagdalpur District m Bastar – 494001 6 A.C.N Cable Pvt. Ltd. Trade Center, No. 29/4, Karnataka Company 9/44/2013-BP&L 21-07-2015 20-07-2025 80428 84888 [email protected] 4th Floor, Race Course Road, 95380 67831 [email protected] Banglore – 560001 080 4288-4288 7 Aadhar Digital Vision Pvt. 37/19, Ayalur Muthiah Street, Tamil Nadu Company 9/56/2012-BP&L 21-02-2014 20-02-2024 98409 03060 [email protected] Ltd Kondithope, Chennai - 600079 94449 99763 [email protected] 8 Aadhishakti Digital Plot No. -

Market Preview Domestic Indices Market Snapshot Global Indices
24-DEC-2020 Domestic Indices Market wrap up Domestic indices Key domestic indices ended with strong gains on Wednesday, index Close Prv close %Chg supported by rally in IT stocks. The Nifty closed above the crucial NIFTY 50 13,601.1 13,466.3 1.00 NIFTY SMALLCAP 50 3425.5 3327.15 2.96 13,600 mark. All the sectoral indices on the NSE ended in the NIFTY MIDCAP 50 5,757.5 5,598.5 2.84 green. Positive global cues boosted sentiment.The BSE Sensex NIFTY SMALLCAP 250 5881.8 5718.9 2.85 rallied 437.49 points to 46,444.18. The Nifty 50 index added NIFTY BANK 29,883.3 29,626.0 0.87 134.80 points or 1% to 13,601.10.HUL, ITC and Reliance NIFTY NEXT 50 31949.8 31417.3 1.69 NIFTY METAL 3,134.1 3,079.7 1.77 Industries advanced.The broader market rallied. The BSE Mid- INDIA VIX 20.5 21.99 -6.77 Cap index rose 2.40% and the BSE Small-Cap index gained Global indices 2.65%.Buyers outpaced sellers. On the BSE, 2,296 shares rose and 650 shares fell. A total of 151 shares were unchanged. index Close Prv close %Chg Global Market NASDAQ 11,854.0 11,849.3 0.04% The S&P 500 closed barely in positive territory on Wednesday as DOW 28,323.4 28,391.4 -0.24% CAC 40 5,495.0 5,407.1 1.60% an expected stimulus deal and falling jobless claims prompted DAX 11,137.0 10,919.8 1.95% investors to put their money into sectors most likely to benefit NIKKEI 25,527.0 24,985.8 2.12% from the economy re-opening when it recovers from the global HANG SENG 26,016.2 25,709.2 1.18% health crisis.The Dow Jones Industrial Average rose 114.32 NYSE 2,072.2 2,077.2 -0.24% FTSE 1,524.3 1,519.6 0.31% points, or 0.38%, to 30,129.83, the S&P 500 gained 2.75 points, or 0.07%, to 3,690.01 and the Nasdaq Composite dropped 36.80 As on 8.00 IST points, or 0.29%, to 12,771.11.Asian shares were set to rise on NiftyMarket Watch Snapshot Thursday ahead of the Christmas break, as global investors cheered a potential Brexit deal and economic recovery prospects, largely ignoring U.S. -

20130118104629Small41.Pdf
Crores of Indian households. A number of family members. Multiple needs. One choice. Joint promoters’ statement “A presence in every house, a recall in every mind – ‘ghar ghar mein Emami’ – has been our driving inspiration since inception” Joint promoters Shri R.S. Agarwal and Shri R.S. Goenka, highlight the Company’s direction and potential There are a number of numerical another year of our successful growth. Emami and growth parameters that one can use to Our performance improved While apparently it appears that 2007- demonstrate that we performed significantly as 2007-08 was a good 08 was tilted towards the industry, the creditably during the financial year year with our topline and bottomline fact remains that we confronted the 2007-08, but the one that stands the growing by 13% and 41% respectively. biggest challenges in terms of an test of industry, analysts and company We feel that there was a sustained unprecedented increase in the cost of is our performance compared with the growth in per capita income, riding on raw materials, a strong price- broad industry average. For instance, the ripple effect of India’s economic sensitivity across most product the broad FMCG industry in India grew growth which benefited a bigger categories, a greater need for wider at a CAGR of about 6% in the last concentration of households. As there and deeper distribution to reach three years; Emami grew at a CAGR was a perceptible increase in people’s customers and the ever-lingering of 25%. aspirations and purchasing power, our threat from counterfeit and This stellar performance sends multiple advertising and brand positioning unorganised products.