Evaluation of Portosystemic Collaterals by SPECT Imaging After Endoscopic Variceal Sclerotherapy: Usefulness for Predicting Recurrence
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Splenic Artery Embolization for the Treatment of Gastric Variceal Bleeding Secondary to Splenic Vein Thrombosis Complicated by Necrotizing Pancreatitis: Report of a Case
Hindawi Publishing Corporation Case Reports in Medicine Volume 2016, Article ID 1585926, 6 pages http://dx.doi.org/10.1155/2016/1585926 Case Report Splenic Artery Embolization for the Treatment of Gastric Variceal Bleeding Secondary to Splenic Vein Thrombosis Complicated by Necrotizing Pancreatitis: Report of a Case Hee Joon Kim, Eun Kyu Park, Young Hoe Hur, Yang Seok Koh, and Chol Kyoon Cho Department of Surgery, Chonnam National University Medical School, Gwangju, Republic of Korea Correspondence should be addressed to Chol Kyoon Cho; [email protected] Received 11 August 2016; Accepted 1 November 2016 Academic Editor: Omer Faruk Dogan Copyright © 2016 Hee Joon Kim et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Splenic vein thrombosis is a relatively common finding in pancreatitis. Gastric variceal bleeding is a life-threatening complication of splenic vein thrombosis, resulting from increased blood flow to short gastric vein. Traditionally, splenectomy is considered the treatment of choice. However, surgery in necrotizing pancreatitis is dangerous, because of severe inflammation, adhesion, and bleeding tendency. In the Warshaw operation, gastric variceal bleeding is rare, even though splenic vein is resected. Because the splenic artery is also resected, blood flow to short gastric vein is not increased problematically. Herein, we report a case of gastric variceal bleeding secondary to splenic vein thrombosis complicated by necrotizing pancreatitis successfully treated with splenic artery embolization. Splenic artery embolization could be the best treatment option for gastric variceal bleeding when splenectomy is difficult such as in case associated with severe acute pancreatitis or associated with severe adhesion or in patients withhigh operation risk. -
Arteries and Veins) of the Gastrointestinal System (Oesophagus to Anus)
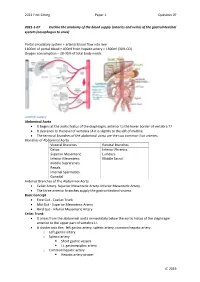
2021 First Sitting Paper 1 Question 07 2021-1-07 Outline the anatomy of the blood supply (arteries and veins) of the gastrointestinal system (oesophagus to anus) Portal circulatory system + arterial blood flow into liver 1100ml of portal blood + 400ml from hepatic artery = 1500ml (30% CO) Oxygen consumption – 20-35% of total body needs Arterial Supply Abdominal Aorta • It begins at the aortic hiatus of the diaphragm, anterior to the lower border of vertebra T7. • It descends to the level of vertebra L4 it is slightly to the left of midline. • The terminal branches of the abdominal aorta are the two common iliac arteries. Branches of Abdominal Aorta Visceral Branches Parietal Branches Celiac. Inferior Phrenics. Superior Mesenteric. Lumbars Inferior Mesenteric. Middle Sacral. Middle Suprarenals. Renals. Internal Spermatics. Gonadal Anterior Branches of The Abdominal Aorta • Celiac Artery. Superior Mesenteric Artery. Inferior Mesenteric Artery. • The three anterior branches supply the gastrointestinal viscera. Basic Concept • Fore Gut - Coeliac Trunk • Mid Gut - Superior Mesenteric Artery • Hind Gut - Inferior Mesenteric Artery Celiac Trunk • It arises from the abdominal aorta immediately below the aortic hiatus of the diaphragm anterior to the upper part of vertebra LI. • It divides into the: left gastric artery, splenic artery, common hepatic artery. o Left gastric artery o Splenic artery ▪ Short gastric vessels ▪ Lt. gastroepiploic artery o Common hepatic artery ▪ Hepatic artery proper JC 2019 2021 First Sitting Paper 1 Question 07 • Left hepatic artery • Right hepatic artery ▪ Gastroduodenal artery • Rt. Gastroepiploic (gastro-omental) artery • Sup pancreatoduodenal artery • Supraduodenal artery Oesophagus • Cervical oesophagus - branches from inferior thyroid artery • Thoracic oesophagus - branches from bronchial arteries and aorta • Abd. -

Dr. ALSHIKH YOUSSEF Haiyan
Dr. ALSHIKH YOUSSEF Haiyan General features The peritoneum is a thin serous membrane Consisting of: 1- Parietal peritoneum -lines the ant. Abdominal wall and the pelvis 2- Visceral peritoneum - covers the viscera 3- Peritoneal cavity - the potential space between the parietal and visceral layer of peritoneum - in male, is a closed sac - but in the female, there is a communication with the exterior through the uterine tubes, the uterus, and the vagina ▪ Peritoneum cavity divided into Greater sac Lesser sac Communication between them by the epiploic foramen The peritoneum The peritoneal cavity is the largest one in the body. Divided into tow sac : .Greater sac; extends from diaphragm down to the pelvis. Lesser Sac .Lesser sac or omental bursa; lies behind the stomach. .Both cavities are interconnected through the epiploic foramen(winslow ). .In male : the peritoneum is a closed sac . .In female : the sac is not completely closed because it Greater Sac communicates with the exterior through the uterine tubes, uterus and vagina. Peritoneum in transverse section The relationship between viscera and peritoneum Intraperitoneal viscera viscera is almost totally covered with visceral peritoneum example, stomach, 1st & last inch of duodenum, jejunum, ileum, cecum, vermiform appendix, transverse and sigmoid colons, spleen and ovary Intraperitoneal viscera Interperitoneal viscera Retroperitoneal viscera Interperitoneal viscera Such organs are not completely wrapped by peritoneum one surface attached to the abdominal walls or other organs. Example liver, gallbladder, urinary bladder and uterus Upper part of the rectum, Ascending and Descending colon Retroperitoneal viscera some organs lie on the posterior abdominal wall Behind the peritoneum they are partially covered by peritoneum on their anterior surfaces only Example kidney, suprarenal gland, pancreas, upper 3rd of rectum duodenum, and ureter, aorta and I.V.C The Peritoneal Reflection The peritoneal reflection include: omentum, mesenteries, ligaments, folds, recesses, pouches and fossae. -

Vessels and Circulation
CARDIOVASCULAR SYSTEM OUTLINE 23.1 Anatomy of Blood Vessels 684 23.1a Blood Vessel Tunics 684 23.1b Arteries 685 23.1c Capillaries 688 23 23.1d Veins 689 23.2 Blood Pressure 691 23.3 Systemic Circulation 692 Vessels and 23.3a General Arterial Flow Out of the Heart 693 23.3b General Venous Return to the Heart 693 23.3c Blood Flow Through the Head and Neck 693 23.3d Blood Flow Through the Thoracic and Abdominal Walls 697 23.3e Blood Flow Through the Thoracic Organs 700 Circulation 23.3f Blood Flow Through the Gastrointestinal Tract 701 23.3g Blood Flow Through the Posterior Abdominal Organs, Pelvis, and Perineum 705 23.3h Blood Flow Through the Upper Limb 705 23.3i Blood Flow Through the Lower Limb 709 23.4 Pulmonary Circulation 712 23.5 Review of Heart, Systemic, and Pulmonary Circulation 714 23.6 Aging and the Cardiovascular System 715 23.7 Blood Vessel Development 716 23.7a Artery Development 716 23.7b Vein Development 717 23.7c Comparison of Fetal and Postnatal Circulation 718 MODULE 9: CARDIOVASCULAR SYSTEM mck78097_ch23_683-723.indd 683 2/14/11 4:31 PM 684 Chapter Twenty-Three Vessels and Circulation lood vessels are analogous to highways—they are an efficient larger as they merge and come closer to the heart. The site where B mode of transport for oxygen, carbon dioxide, nutrients, hor- two or more arteries (or two or more veins) converge to supply the mones, and waste products to and from body tissues. The heart is same body region is called an anastomosis (ă-nas ′tō -mō′ sis; pl., the mechanical pump that propels the blood through the vessels. -

Portal Hypertensionand Its Radiological Investigation
Postgrad Med J: first published as 10.1136/pgmj.39.451.299 on 1 May 1963. Downloaded from POSTGRAD. MED. J. (I963), 39, 299 PORTAL HYPERTENSION AND ITS RADIOLOGICAL INVESTIGATION J. H. MIDDLEMISS, M.D., F.F.R., D.M.R.D. F. G. M. Ross, M.B., B.Ch., B.A.O., F.F.R., D.M.R.D. From the Department of Radiodiagnosis, United Bristol Hospitals PORTAL hypertension is a condition in which there branch of the portal vein but may drain into the right is an blood in the branch. abnormally high pressure Small veins which are present on the serosal surface portal system of veins which eventually leads to of the liver and in the surrounding peritoneal folds splenomegaly and in chronic cases, to haematem- draining the diaphragm and stomach are known as esis and melaena. accessory portal veins. They may unite with the portal The circulation is in that it vein or enter the liver independently. portal unique The hepatic artery arises normally from the coeliac exists between two sets of capillaries, i.e. the axis but it may arise as a separate trunk from the aorta. capillaries of the spleen, pancreas, gall-bladder It runs upwards and to the right and divides into a and most of the gastro-intestinal tract on the left and right branch before entering the liver at the one hand and the sinusoids of the liver on the porta hepatis. The venous return starts as small thin-walled branches other hand. The liver parallels the lungs in that in the centre of the lobules in the liver. -

The Diameter of the Originating Vein Determines Esophageal and Gastric Fundic Varices in Portal Hypertension Secondary to Posthepatitic Cirrhosis
CLINICS 2012;67(6):609-614 DOI:10.6061/clinics/2012(06)11 CLINICAL SCIENCE The diameter of the originating vein determines esophageal and gastric fundic varices in portal hypertension secondary to posthepatitic cirrhosis Hai-ying Zhou, Tian-wu Chen, Xiao-ming Zhang, Li-ying Wang, Li Zhou, Guo-li Dong, Nan-lin Zeng, Hang Li, Xiao-li Chen, Rui Li Sichuan Key Laboratory of Medical Imaging, and Department of Radiology, Affiliated Hospital of North Sichuan Medical College, Sichuan/China. OBJECTIVE: The aim of this study was to determine whether and how the diameter of the vein that gives rise to the inflowing vein of the esophageal and gastric fundic varices secondary to posthepatitic cirrhosis, as measured with multidetector-row computed tomography, could predict the varices and their patterns. METHODS: A total of 106 patients with posthepatitic cirrhosis underwent multidetector-row computed tomography. Patients with and without esophageal and gastric fundic varices were enrolled in Group 1 and Group 2, respectively. Group 1 was composed of Subgroup A, consisting of patients with varices, and Subgroup B consisted of patients with varices in combination with portal vein-inferior vena cava shunts. The diameters of the originating veins of veins entering the varices were reviewed and statistically analyzed. RESULTS: The originating veins were the portal vein in 8% (6/75) of patients, the splenic vein in 65.3% (49/75) of patients, and both the portal and splenic veins in 26.7% (20/75) of patients. The splenic vein diameter in Group 1 was larger than that in Group 2, whereas no differences in portal vein diameters were found between groups. -

Normal Gross and Histologic Features of the Gastrointestinal Tract
NORMAL GROSS AND HISTOLOGIC 1 FEATURES OF THE GASTROINTESTINAL TRACT THE NORMAL ESOPHAGUS left gastric, left phrenic, and left hepatic accessory arteries. Veins in the proximal and mid esopha- Anatomy gus drain into the systemic circulation, whereas Gross Anatomy. The adult esophagus is a the short gastric and left gastric veins of the muscular tube measuring approximately 25 cm portal system drain the distal esophagus. Linear and extending from the lower border of the cri- arrays of large caliber veins are unique to the distal coid cartilage to the gastroesophageal junction. esophagus and can be a helpful clue to the site of It lies posterior to the trachea and left atrium a biopsy when extensive cardiac-type mucosa is in the mediastinum but deviates slightly to the present near the gastroesophageal junction (4). left before descending to the diaphragm, where Lymphatic vessels are present in all layers of the it traverses the hiatus and enters the abdomen. esophagus. They drain to paratracheal and deep The subdiaphragmatic esophagus lies against cervical lymph nodes in the cervical esophagus, the posterior surface of the left hepatic lobe (1). bronchial and posterior mediastinal lymph nodes The International Classification of Diseases in the thoracic esophagus, and left gastric lymph and the American Joint Commission on Cancer nodes in the abdominal esophagus. divide the esophagus into upper, middle, and lower thirds, whereas endoscopists measure distance to points in the esophagus relative to the incisors (2). The esophagus begins 15 cm from the incisors and extends 40 cm from the incisors in the average adult (3). The upper and lower esophageal sphincters represent areas of increased resting tone but lack anatomic landmarks; they are located 15 to 18 cm from the incisors and slightly proximal to the gastroesophageal junction, respectively. -

Color Doppler Evaluation of Left Gastric Vein Hemodynamics in Cirrhosis
Published online: 2021-08-02 ABDOMINAL Color Doppler evaluation of left gastric vein hemodynamics in cirrhosis with portal hypertension and its correlation with esophageal varices and variceal bleed Subathra Adithan, Bhuvaneswari Venkatesan, Elangovan Sundarajan, Vikram Kate1, Raja Kalayarasan1 Departments of Radiodiagnosis and 1Surgery, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Pondicherry - 605 006, India Correspondence: Dr. Subathra Adithan, Department of Radiodiagnosis, JIPMER, Dhanvantri Nagar, Pondicherry, India. E-mail: [email protected] Abstract Aim: The purpose of this study was to assess the value of Doppler evaluation of left gastric vein hemodynamics when monitoring portal hypertension patients, by correlating Doppler ultrasonography (USG) parameters with the severity of esophageal varices and occurrence of variceal bleeding. Methods: This study was carried out on 100 patients using Doppler USG and endoscopy. Forty- seven of these were patients with cirrhosis with portal hypertension, who had not had a recent variceal bleed (group 1) and 26 were patients with cirrhosis with portal hypertension, with a recent history of bleeding (group 2). The control group comprised of 27 subjects who did not have liver disease or varices on endoscopy (group 3). The hemodynamic parameters, namely the diameter of the left gastric vein and the direction and flow velocity in the vessel, were compared in these groups, with the grade of esophageal varices. Results: Hepatofugal flow velocity in the left gastric vein was higher in patients with large-sized varices compared to those patients with small-sized varices (P < 0.001). The left gastric vein hepatofugal flow velocity was higher in patients with a recent variceal bleed than in those patients without a history of a recent variceal bleed (P < 0.0149). -

Residency Essentials Full Curriculum Syllabus
RESIDENCY ESSENTIALS FULL CURRICULUM SYLLABUS Please review your topic area to ensure all required sections are included in your module. You can also use this document to review the surrounding topics/sections to ensure fluidity. Click on the topic below to jump to that page. Clinical Topics • Gastrointestinal • Genitourinary • Men’s Health • Neurological • Oncology • Pain Management • Pediatrics • Vascular Arterial • Vascular Venous • Women’s Health Requisite Knowledge • Systems • Business and Law • Physician Wellness and Development • Research and Statistics Fundamental • Clinical Medicine • Intensive Care Medicine • Image-guided Interventions • Imaging and Anatomy Last revised: November 4, 2019 Gastrointestinal 1. Portal hypertension a) Pathophysiology (1) definition and normal pressures and gradients, MELD score (2) Prehepatic (a) Portal, SMV or Splenic (i) thrombosis (ii) stenosis (b) Isolated mesenteric venous hypertension (c) Arterioportal fistula (3) Sinusoidal (intrahepatic) (a) Cirrhosis (i) ETOH (ii) Non-alcoholic fatty liver disease (iii) Autoimmune (iv) Viral Hepatitis (v) Hemochromatosis (vi) Wilson's disease (b) Primary sclerosing cholangitis (c) Primary biliary cirrhosis (d) Schistosomiasis (e) Infiltrative liver disease (f) Drug/Toxin/Chemotherapy induced chronic liver disease (4) Post hepatic (a) Budd Chiari (Primary secondary) (b) IVC or cardiac etiology (5) Ectopic perianastomotic and stomal varices (6) Splenorenal shunt (7) Congenital portosystemic shunt (Abernethy malformation) b) Measuring portal pressure (1) Direct -

Studies on Laparoscopic Gastric Surgery in Korea
Surgical Anatomy of UGI Seung-Wan Ryu Keimyung University, Korea Location • The stomach is a dilated part of the alimentary canal. • It is located in the upper part of the abdomen. • It extends from beneath the left costal margin into the epigastric and umbilical regions. • Position of the stomach varies with body habitues PARTS 2 Orifices: Cardiac orifice Pyloric orifice 2 Borders: Greater curvature Lesser curvature 2 Surfaces: Anterior surface Posterior surface 3 Parts: Fundus Body Pylorus: FUNDUS • Dome-shaped • Located to the left of the cardiac orifice • Usually full of gas. • In X-Ray film it appears black BODY • Extends from: The level of the fundus to The level of Incisura Angularis a constant notch on the lesser curvature LESSER CURVATURE • Forms the right border of the stomach. • Extends from the cardiac orifice to the pylorus. • Attached to the liver by the lesser omentum. GREATER CURVATURE • Forms the left border of the stomach. • Extends from the cardiac orifice to the pylorus • Its upper part is attached to the spleen by gastrosp lenic ligament • Its lower part is attached to the transverse colon by the greater omentum. ANTERIOR RELATIONS • Anterior abdominal wall • Left costal margin • Left pleura & lung • Diaphragm • Left lobe of the liver POSTERIOR RELATIONS • Stomach Bed: • Peritoneum (Lesser sac) • Left crus of diaphragm • Left suprarenal gland • Part of left kidney • Spleen • Splenic artery • Pancreas • Transverse mesocolon • They are separated from the stomach by Peritoneum (Lesser sac except the spleen) Blood Supply ARTERIES • 5 arteries: • As it is derived from the foregut all are branches of the celiac trunk • 1- Left gastric artery: It is a branch of celiac artery. -

Venous Drainage from the Tail of the Pancreas to the Lienal Vein and Its Relationship with the Distal Splenorenal Shunt Selectivity1
20 – ORIGINAL ARTICLE Surgical Anatomy Venous drainage from the tail of the pancreas to the lienal vein and its relationship with the distal splenorenal shunt selectivity1 Drenagem venosa da cauda do pâncreas para a veia lienal e sua relação com a seletividade da anastomose esplenorrenal Cláudio PirasI, Danilo Nagib Salomão PauloII, Isabel Cristina Andreatta Lemos PauloIII, Hildegardo RodriguesIV, Alcino Lázaro da SilvaV I Associate Professor, Department of Surgery, School of Sciences, EMESCAM, Espirito Santo, Brazil. II Full Professor of Surgery, Department of Surgery, School of Sciences, EMESCAM, Espirito Santo, Brazil. III Associate Professor, Department of Surgery, School of Sciences, EMESCAM, Espirito Santo, Brazil. IV Professor of Anatomy, Department of Morphology, School of Sciences, EMESCAM, Espirito Santo, Brazil. V Emeritus Professor of Surgery, School of Medicine, Federal University of Minas Gerais, Brazil. ABSTRACT Purpose: To identify the veins draining from the pancreatic tail to the lienal vein and its possible relationship with the loss of the distal splenorenal shunt selectivity. Methods: Thirty eight human blocks including stomach, duodenum, spleen, colon and pancreas, removed from fresh corpses, were studied with the replenish and corrosion technique, using vinilic resin and posterior corrosion of the organic tissue with commercial hydrochloric acid, in order to study the lienal vein and its tributaries. Results: The number of veins flowing directly to the splenic vein varied from seven to twenty two (14.52 ± 3.53). Pancreatic branches of the pancreatic tail flowing to the segmentary veins of the spleen were found in 25 of the anatomical pieces studied (65.79%). These branches varied from one to four, predominating one branch (60%) and two branches (24%). -

Thieme: MR Imaging Abdomen Pelvis
Focal Liver Lesions 11 Right hepatic lobe Left hepatic lobe Inferior vena cava Right hepatic vein Left hepatic vein Segment I (caudate lobe) Middle hepatic vein Segment II Segment VIII Segment III Segment IV Left umbilical vein Segment VII Portal vein Segment VI Segment V Left portal vein Right portal vein Fig. 1.8 Segmental anatomy of the liver. If a hepatobiliary contrast medium is used to differentiate MRI Appearance of Pathologic Entities hepatocellular tumors (focal nodular hyperplasia, well- differentiated hepatocellular carcinoma), additional de- Most benign and malignant liver tumors are hypointense layed images should be obtained no earlier than 2 h after on T1w images and hyperintense on T2w images. Cysts, contrast injection. hemangiomas, and metastases from neuroendocrine tu- mors are visualized with high contrast, as are intra- tumoral necrosis and abscesses. The vast majority of other MRI Appearance of Normal Anatomy liver metastases and cholangiocarcinoma (CCA) are less conspicuous on both T1w and T2w images. Liver tumors The normal liver is of uniform signal intensity, which is thatareisointenseornearlyisointensetonormalliver higher than that of the spleen on T1w images and lower parenchyma are focal nodular hyperplasia (FNH) as well on T2w images. Hepaticvessels appear dark on T1w im- as an occasional adenoma or hepatocellular carcinoma ages due to flow-related signal loss (see Fig. 1.2). They are (HCC) (Table 1.4). T1w sequences contribute little to lesion also dark on T2w images unless gradient moment nulling characterization, except for cysts, which are clearly iden- (flow compensation) is used, in which case intrahepatic tified as sharply demarcated lesions of very low signal vessels are bright.