Polyploidy and the Sexual System: What Can We Learn from Mercurialis Annua?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution of Genetic Systems in Filamentous Ascomycetes
Evolution of Genetic Systems in Filamentous Ascomycetes Evolutie van genetische systemen in hyphenvormende zakjeszwammen 0000 0513 3836 Promotor: dr. R.F. Hoekstra hoogleraar in de populatie- en kwantitatieve genetica fjtfoiißi f ßin Maarten J. Nauta Evolution of Genetic Systems in Filamentous Ascomycetes Proefschrift ter verkrijging van de graad van doctor in de landbouw- en milieuwetenschappen op gezag van de rector magnificus, dr. C.M. Karssen, in het openbaar te verdedigen op woensdag 12januar i 1994 des namiddags te vier uur in de Aula van de Landbouwuniversiteit te Wageningen. 15 0 S(p^ZJ> These investigations were supported by the Netherlands Organization for Scientific Research (N.W.O.). BibUt/FHEEK LAMDbOirWUNIVERSITEJi. WAGE NINGE N CIP-GEGEVENS KONINKLIJKE BIBLIOTHEEK, DEN HAAG Nauta, Maarten J. Evolution of genetic systems in filamentous ascomycetes / Maarten J. Nauta. - [ S.l. : s.n.]. -111 . Thesis Wageningen. - With ref. - With summary in Dutch. ISBN 90-5485-199-6 Subject headings: population genetics / ascomycetes. omslagontwerp: Ernst van Cleef foto omslag: Barrages tussen verschillende stammen van Podospora anserina als gevolg van vegetatieve incompatibiliteit. (met dank aan Inge Haspels) aan mijn ouders Voorwoord Dit proefschrift is het resultaat van vier jaar onderzoek, verricht bij de vakgroep Erfelijkheidsleer van de Landbouwuniversiteit in Wageningen. In zekere zin valt zo'n proefschrift te vergelijken met een levend wezen. Uit de genetica is bekend dat de verschijningsvorm van elk levend wezen tot stand komt door een combinatie van erfelijke aanleg en invloeden uit de omgeving. Voor een proefschrift geldt eigenlijk hetzelfde: Zowel het werk van de auteur, als de bijdragen van zijn omgeving zijn onontbeerlijk om tot een verschijningsvorm te komen. -

Evolutionary Pathways to Self-Fertilization in a Tristylous Plant
Review BlackwellOxford,NPHNew0028-646X1469-8137©293710.1111/j.1469-8137.2009.02937.xJune0546???556???ResearchXX The 2009Phytologist Authors UK Review Publishing (2009). Ltd Journal compilation © New Phytologist (2009) Research reviewXX Evolutionary pathways to self- fertilization in a tristylous plant species Author for correspondence: Spencer C. H. Barrett, Rob W. Ness and Mario Vallejo-Marín Spencer C. H. Barrett Department of Ecology and Evolutionary Biology, University of Toronto, 25 Willcocks St, Toronto, Tel: +1 416 978 5603 Email: [email protected] Ontario, Canada, M5S 3B2 Received: 23 April 2009 Accepted: 20 May 2009 Summary New Phytologist (2009) 183: 546–556 Evolutionary transitions from outcrossing to selfing occur commonly in heterostylous doi: 10.1111/j.1469-8137.2009.02937.x genera. The morphological polymorphisms that characterize heterostyly provide opportunities for different pathways for selfing to evolve. Here, we investigate the Key words: developmental instability, origins and pathways by which selfing has evolved in tristylous Eichhornia paniculata Eichhornia, herkogamy, heterostyly, multiple by providing new evidence based on morphology, DNA sequences and genetic analysis. origins, pathways to self-fertilization. The primary pathway from outcrossing to selfing involves the stochastic loss of the short-styled morph (S-morph) from trimorphic populations, followed by the spread of selfing variants of the mid-styled morph (M-morph). However, the discovery of selfing variants of the long-styled morph (L-morph) in Central America indicates a secondary pathway and distinct origin for selfing. Comparisons of multi-locus nucleo- tide sequences from 27 populations sampled from throughout the geographical range suggest multiple transitions to selfing. Genetic analysis of selfing variants of the L- and M-morphs demonstrates recessive control of the loss of herkogamy, although the number of factors appears to differ between the forms. -
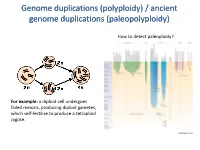
Polyploidy) / Ancient Genome Duplications (Paleopolyploidy
Genome duplications (polyploidy) / ancient genome duplications (paleopolyploidy) How to detect paleoploidy? For example: a diploid cell undergoes failed meiosis, producing diploid gametes, which self-fertilize to produce a tetraploid zygote. Timing of duplication by trees (phylogenetic timing) Phylogenetic timing of duplicates b Paramecium genome duplications Comparison of two scaffolds originating from a common ancestor at the recent WGD Saccharomyces cerevisiae Just before genome duplication Just after genome duplication More time after genome duplication Unaligned view (removing gaps just like in cerev has occurred) Saccharomyces cerevisiae Problem reciprocal gene loss (extreme case); how to solve? Problem reciprocal gene loss (extreme case); how to solve? Just before genome duplication Outgroup! Just after genome duplication Outgroup Just after genome duplication Outgroup More time after genome duplication Outgroup Problem (extreme case); how to solve? Outgroup Outgroup Outgroup Outgroup Outgroup Using other genomes Wong et al. 2002 PNAS Centromeres Vertebrate genome duplication Nature. 2011 Apr 10. [Epub ahead of print] Ancestral polyploidy in seed plants and angiosperms. Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, Tomsho LP, Hu Y, Liang H, Soltis PS, Soltis DE, Clifton SW, Schlarbaum SE, Schuster SC, Ma H, Leebens-Mack J, Depamphilis CW. Flowering plants Flowering MOSS Vertebrates Teleosts S. serevisiae and close relatives Paramecium Reconstructed map of genome duplications allows unprecedented mapping -

The Diversity of Plant Sex Chromosomes Highlighted Through Advances in Genome Sequencing
G C A T T A C G G C A T genes Review The Diversity of Plant Sex Chromosomes Highlighted through Advances in Genome Sequencing Sarah Carey 1,2 , Qingyi Yu 3,* and Alex Harkess 1,2,* 1 Department of Crop, Soil, and Environmental Sciences, Auburn University, Auburn, AL 36849, USA; [email protected] 2 HudsonAlpha Institute for Biotechnology, Huntsville, AL 35806, USA 3 Texas A&M AgriLife Research, Texas A&M University System, Dallas, TX 75252, USA * Correspondence: [email protected] (Q.Y.); [email protected] (A.H.) Abstract: For centuries, scientists have been intrigued by the origin of dioecy in plants, characterizing sex-specific development, uncovering cytological differences between the sexes, and developing theoretical models. Through the invention and continued improvements in genomic technologies, we have truly begun to unlock the genetic basis of dioecy in many species. Here we broadly review the advances in research on dioecy and sex chromosomes. We start by first discussing the early works that built the foundation for current studies and the advances in genome sequencing that have facilitated more-recent findings. We next discuss the analyses of sex chromosomes and sex-determination genes uncovered by genome sequencing. We synthesize these results to find some patterns are emerging, such as the role of duplications, the involvement of hormones in sex-determination, and support for the two-locus model for the origin of dioecy. Though across systems, there are also many novel insights into how sex chromosomes evolve, including different sex-determining genes and routes to suppressed recombination. We propose the future of research in plant sex chromosomes should involve interdisciplinary approaches, combining cutting-edge technologies with the classics Citation: Carey, S.; Yu, Q.; to unravel the patterns that can be found across the hundreds of independent origins. -

Genetic Diversity in Partially Selfing Populations with the Stepping-Stone Structure
Heredity 77 (1996) 469—475 Received 20 October 1995 Genetic diversity in partially selfing populations with the stepping-stone structure HIDENORI TACHIDA*t & HIROSHI YOSHIMARU tDepartment of Biology, Faculty of Science, Kyushu University 33, Fukuoka 812, Japan and tPopulation Genetics Laboratory, Forestry and Forest Products Research Institute, Tsukuba, Ibaraki 305, Japan Amethod to compute identity coefficients of two genes in the stepping-stone model with partial selfing is developed. The identity coefficients in partially selfing populations are computed from those in populations without selfing as functions of s (selfing rate), m (migra- tion rate), N (subpopulation size), n (number of subpopulations) and u (mutation rate). For small m, 1/N and u, it is shown that approximate formulae for the identity coefficients of two genes from different individuals are the same as those in random mating populations if we replace N in the latter with N(1 —s12).Thus,the effects of selfing on genetic variability are summarized as reducing variation within subpopulations and increasing differentiation among subpopulations by reducing the subpopulation size. The extent of biparental inbreeding as measured by the genotypic correlation between truly outcrossed mates was computed in the one-dimensional stepping-stone model. The correlation was shown to be independent of the selfing rate and starts to fall off as the migration rate increases when mN is larger than 0.1. Keywords:biparentalinbreeding, fixation index, genetic variability, geographical differentia- tion, selfing, stepping-stone model. Introduction Model analysis Manyplant species are partially self-fertilizing Genesare defined to be identical by descent if they (Brown, 1990; Murawski & Hamrick, 1991, 1992; have a common ancestor gene and there is no muta- Kitamura et a!., 1994). -

Inbreeding Depression in Two Populations of Arenaria Uni¯Ora (Caryophyllaceae) with Contrasting Mating Systems
Heredity 86 (2001) 184±194 Received 16 November 1999, accepted 17 October 2000 Inbreeding depression in two populations of Arenaria uni¯ora (Caryophyllaceae) with contrasting mating systems LILA FISHMAN Department of Ecology and Evolutionary Biology, Princeton University, Princeton, NJ 08544 U.S.A. I used parallel family-structured crossing designs to investigate the relative performance of self and outcross progeny in sel®ng and predominantly outcrossing populations of the annual plant Arenaria uni¯ora. The selfer population experienced much lower inbreeding depression (d 0.05 0.02 SE) than the outcrossers (d 0.19 0.02 SE). The negative association between genetic load and sel®ng rate suggests that purgable partially recessive alleles are the primary source of inbreeding depression, as does its late expression in both populations. Inbreeding depression in the selfer population, which naturally consists of highly inbred lines, was used to calculate the mean dominance (h 0.33) and incidence rate (U 0.30) of deleterious mutations. In the outcrosser population, signi®cant variation among individuals in the expression of inbreeding depression may re¯ect lineage-speci®c dierences in inbreeding history or, more probably, random variation in mutational load. The low (0.5) inbreeding depression of outcrossers suggests that the maintenance of a mixed mating system in some A. uni¯ora populations and the evolution of nearly cleistogamous self-pollination in others may re¯ect local pollinator-mediated selection for sel®ng rather than the constant 3:2 genetic advantage invoked by many models. Keywords: Arenaria uni¯ora, genetic load, inbreeding depression, mating system, sel®ng. -

Population Genetics of the Wild Yeast Saccharomyces Paradoxus
Copyright 2004 by the Genetics Society of America Population Genetics of the Wild Yeast Saccharomyces paradoxus Louise J. Johnson,*,1 Vassiliki Koufopanou,* Matthew R. Goddard,† Richard Hetherington,* Stefanie M. Scha¨fer*,2 and Austin Burt* *Department of Biological Sciences and †NERC Centre for Population Biology, Imperial College at Silwood Park, Ascot SL5 7PY, United Kingdom Manuscript received November 4, 2002 Accepted for publication September 22, 2003 ABSTRACT Saccharomyces paradoxus is the closest known relative of the well-known S. cerevisiae and an attractive model organism for population genetic and genomic studies. Here we characterize a set of 28 wild isolates from a 10-km2 sampling area in southern England. All 28 isolates are homothallic (capable of mating-type switching) and wild type with respect to nutrient requirements. Nine wild isolates and two lab strains of S. paradoxus were surveyed for sequence variation at six loci totaling 7 kb, and all 28 wild isolates were then genotyped at seven polymorphic loci. These data were used to calculate nucleotide diversity and number of segregating sites in S. paradoxus and to investigate geographic differentiation, population Extensive incompatibilities .%0.3ف structure, and linkage disequilibrium. Synonymous site diversity is between gene genealogies indicate frequent recombination between unlinked loci, but there is no evidence of recombination within genes. Some localized clonal growth is apparent. The frequency of outcrossing relative to inbreeding is estimated at 1.1% on the basis of heterozygosity. Thus, all three modes of reproduction known in the lab (clonal replication, inbreeding, and outcrossing) have been important in molding genetic variation in this species. -

Clonal Reproduction in Fungi COLLOQUIUM
PAPER Clonal reproduction in fungi COLLOQUIUM John W. Taylora,1, Christopher Hann-Sodena, Sara Brancoa, Iman Sylvaina, and Christopher E. Ellisonb Departments of aPlant and Microbial Biology and bIntegrative Biology, University of California, Berkeley, CA 94720 Edited by John C. Avise, University of California, Irvine, CA, and approved April 2, 2015 (received for review February 17, 2015) Research over the past two decades shows that both recombina- Clearly, mycologists have more work to do with these extremely tion and clonality are likely to contribute to the reproduction of all important fungi. fungi. This view of fungi is different from the historical and still commonly held view that a large fraction of fungi are exclusively Population Genetic Evidence for Recombination clonal and that some fungi have been exclusively clonal for Evidence that clonality was not limited to Glomales but instead hundreds of millions of years. Here, we first will consider how was widespread in fungi came from the oft-cited statistic that these two historical views have changed. Then we will examine 20% of fungi are asexual (12). This fraction represented the the impact on fungal research of the concept of restrained re- number of fungi for which sexual reproduction had not been combination [Tibayrenc M, Ayala FJ (2012) Proc Natl Acad Sci USA observed or was rarely observed. At a time when observation of 109 (48):E3305–E3313]. Using animal and human pathogenic fungi, the sexual morphology of a fungus was required for its classifi- we examine extrinsic restraints on recombination associated with cation, these fungi were classified in the Deuteromycota, apart bottlenecks in genetic variation caused by geographic dispersal from sexual fungi. -

Evolution of Mating Systems: Outcrossing Versus Selfing Spencer C
In The Princeton Guide to Evolution ed. J. Losos. Princeton University Press, Princeton, New Jersey, pp. 356-362. IV.8 Evolution of Mating Systems: Outcrossing versus Selfing Spencer C. H. Barrett OUTLINE that transitions to predominant selfing have occurred on numerous occasions, although selfing lineages are often 1. Definitions and measurement short-lived because of the negative effects of selfing on 2. Variation in mating patterns the genome. 3. Evolution of self-fertilization 4. Mechanisms of selection 5. The problem of mixed mating 6. Evolutionary history GLOSSARY Floral Design and Display. The morphological features of Mating systems vary enormously among groups of or- flowers and inflorescences that influence pollen dis- ganisms. This has led to diverse definitions and ap- persal and mating patterns in flowering plants; floral proaches for investigating their evolution and main- design involves characteristics of individual flowers, tenance. Animal mating systems are characterized by including their size, structure, color, and the spatial different patterns of parental investment in offspring and temporal presentation of female (pistil) and male and variation in the extent to which sexual selection (stamens) sex organs, and floral display concerns shapes male and female traits (see chapter VII.4). A the number of open flowers on a plant and their ar- primary focus of most studies is determining the causes rangement within and among inflorescences. and consequences of variation in mate number for fe- Inbreeding Depression. The reduction in viability and/ males and males. In contrast, in hermaphrodite organ- or fertility of inbred offspring in comparison with isms, particularly plants, the emphasis is largely on de- outbred offspring. -

Allardts Argument Versus Bakerts Contention for the Adaptive
Allard’s argument versus Baker’s contention for the adaptive significance of selfing in a hermaphroditic fish John C. Avise1 and Andrey Tatarenkov Department of Ecology and Evolutionary Biology, University of California, Irvine, CA 92697 Contributed by John C. Avise, October 2, 2012 (sent for review June 29, 2012) Fertilization assurance (Baker’s contention) and multilocus coadap- heterogeneity” and thus “natural selection was the predominant tation (Allard’s argument) are two distinct hypotheses for the integrating force in shaping the specific genetic structure of dif- adaptive significance of self-fertilization in hermaphroditic taxa, ferent local populations as well as the adaptive landscape.” With and both scenarios have been invoked to rationalize isogenicity regard to purported evolutionary benefits of self-fertilization as via incest in various plants and invertebrate animals with predom- a tactical component of a mixed-mating strategy, for shorthand we inant selfing. Here we contrast Allard’s argument and Baker’s con- will refer to this genomic-coadaptation scenario as Allard’s ar- tention as applied to the world’s only known vertebrate that gument for the adaptive significance of selfing. routinely self-fertilizes. We pay special attention to frequencies Soon thereafter, Robert Selander and colleagues published an of locally most common multilocus genotypes in Floridian popula- analogous series of studies documenting multilocus coadaptation tions of the Mangrove Rivulus (Kryptolebias marmoratus). Isoge- in local populations of terrestrial hermaphroditic snails with high nicity patterns in this fish appear inconsistent with Allard’s selfing rates (13–16). Again, specific multilocus suites of alleles argument, thus leaving Baker’s contention as the more plausible appeared to be coadapted to particular ecological conditions and scenario (a result also supported by natural history information for thereby often were driven to moderate or high frequencies in this species). -

Cell Size, Polyploidy, and Sex Determination in Nasonia
Cell size, polyploidy, and sex determination in Nasonia Supervision by: Kelley Leung Contact: [email protected], room 5172.0662; language: English olyploidy is the condition of having more Pthan the usual number of chromosome sets. In many ways polyploidy is detrimental because of problems of infertility and abnormal cell biol- ogy. However, polyploidization has happened many times via whole genome duplication in the evolutionary tree (including for humans!). It may have provided benefits such as addition- al gene copies and increased hardiness. This begs the question, how do polyploids overcome disadvantages to derive benefits? One possible adaptation that has allowed polyploids to persist is cell reduction mech- anisms. Polyploid cells are larger than normal cells. In vertebrate polyploids, cell re- duction mechanisms are used to retain fairly normal overall body size and physiology. However, it is unknown if this also holds for invertebrate polyploids, including insects. We will use the parasitoid wasp system Nasonia vitripennis to investigate whether there are cell reduction mechanisms in insect polyploids. Like all hymenopterans, N. vitripen- nis has a haplodiploid sex determination system: normally, unfertilized eggs become haploid (1n) males and fertilized eggs become diploid (2n) females. However, there are many ways that ploidy levels change. Some are naturally occurring. Others can be created by using RNAi knockdown to obtain null mutants of various sex determination genes. These lines produce haploid (1n) females, diploid males -

Convergent Recombination Cessation Between Mating-Type Genes and Centromeres in Selfing Anther-Smut Fungi
Downloaded from genome.cshlp.org on September 30, 2021 - Published by Cold Spring Harbor Laboratory Press Research Convergent recombination cessation between mating-type genes and centromeres in selfing anther-smut fungi Fantin Carpentier,1,4 Ricardo C. Rodríguez de la Vega,1,4 Sara Branco,1,6 Alodie Snirc,1 Marco A. Coelho,2,7 Michael E. Hood,3,5 and Tatiana Giraud1,5 1Ecologie Systématique Evolution, Bâtiment 360, Univ. Paris-Sud, AgroParisTech, CNRS, Université Paris-Saclay, 91400 Orsay, France; 2UCIBIO-REQUIMTE, Departamento de Ciências da Vida, Faculdade de Ciências e Tecnologia, Universidade NOVA de Lisboa, 2829-516 Caparica, Portugal; 3Department of Biology, Amherst College, Amherst, Massachusetts 01002, USA The degree of selfing has major impacts on adaptability and is often controlled by molecular mechanisms determining mating compatibility. Changes in compatibility systems are therefore important evolutionary events, but their underlying genomic mechanisms are often poorly understood. Fungi display frequent shifts in compatibility systems, and their small genomes fa- cilitate elucidation of the mechanisms involved. In particular, linkage between the pre- and postmating compatibility loci has evolved repeatedly, increasing the odds of gamete compatibility under selfing. Here, we studied the mating-type chromo- somes of two anther-smut fungi with unlinked mating-type loci despite a self-fertilization mating system. Segregation analyses and comparisons of high-quality genome assemblies revealed that these two species displayed linkage between mating-type loci and their respective centromeres. This arrangement renders the same improved odds of gamete compatibility as direct linkage of the two mating-type loci under the automictic mating (intratetrad selfing) of anther-smut fungi.