Homoeologous Shuffling and Chromosome Compensation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
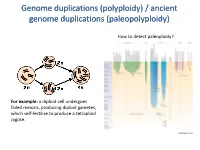
Polyploidy) / Ancient Genome Duplications (Paleopolyploidy
Genome duplications (polyploidy) / ancient genome duplications (paleopolyploidy) How to detect paleoploidy? For example: a diploid cell undergoes failed meiosis, producing diploid gametes, which self-fertilize to produce a tetraploid zygote. Timing of duplication by trees (phylogenetic timing) Phylogenetic timing of duplicates b Paramecium genome duplications Comparison of two scaffolds originating from a common ancestor at the recent WGD Saccharomyces cerevisiae Just before genome duplication Just after genome duplication More time after genome duplication Unaligned view (removing gaps just like in cerev has occurred) Saccharomyces cerevisiae Problem reciprocal gene loss (extreme case); how to solve? Problem reciprocal gene loss (extreme case); how to solve? Just before genome duplication Outgroup! Just after genome duplication Outgroup Just after genome duplication Outgroup More time after genome duplication Outgroup Problem (extreme case); how to solve? Outgroup Outgroup Outgroup Outgroup Outgroup Using other genomes Wong et al. 2002 PNAS Centromeres Vertebrate genome duplication Nature. 2011 Apr 10. [Epub ahead of print] Ancestral polyploidy in seed plants and angiosperms. Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, Tomsho LP, Hu Y, Liang H, Soltis PS, Soltis DE, Clifton SW, Schlarbaum SE, Schuster SC, Ma H, Leebens-Mack J, Depamphilis CW. Flowering plants Flowering MOSS Vertebrates Teleosts S. serevisiae and close relatives Paramecium Reconstructed map of genome duplications allows unprecedented mapping -

The Diversity of Plant Sex Chromosomes Highlighted Through Advances in Genome Sequencing
G C A T T A C G G C A T genes Review The Diversity of Plant Sex Chromosomes Highlighted through Advances in Genome Sequencing Sarah Carey 1,2 , Qingyi Yu 3,* and Alex Harkess 1,2,* 1 Department of Crop, Soil, and Environmental Sciences, Auburn University, Auburn, AL 36849, USA; [email protected] 2 HudsonAlpha Institute for Biotechnology, Huntsville, AL 35806, USA 3 Texas A&M AgriLife Research, Texas A&M University System, Dallas, TX 75252, USA * Correspondence: [email protected] (Q.Y.); [email protected] (A.H.) Abstract: For centuries, scientists have been intrigued by the origin of dioecy in plants, characterizing sex-specific development, uncovering cytological differences between the sexes, and developing theoretical models. Through the invention and continued improvements in genomic technologies, we have truly begun to unlock the genetic basis of dioecy in many species. Here we broadly review the advances in research on dioecy and sex chromosomes. We start by first discussing the early works that built the foundation for current studies and the advances in genome sequencing that have facilitated more-recent findings. We next discuss the analyses of sex chromosomes and sex-determination genes uncovered by genome sequencing. We synthesize these results to find some patterns are emerging, such as the role of duplications, the involvement of hormones in sex-determination, and support for the two-locus model for the origin of dioecy. Though across systems, there are also many novel insights into how sex chromosomes evolve, including different sex-determining genes and routes to suppressed recombination. We propose the future of research in plant sex chromosomes should involve interdisciplinary approaches, combining cutting-edge technologies with the classics Citation: Carey, S.; Yu, Q.; to unravel the patterns that can be found across the hundreds of independent origins. -

63Rd Annual Maize Genetics Meeting Program and Abstracts
63rd Annual Maize Genetics Meeting Program and Abstracts March 8 – March 12, 2021 2nd Virtual Maize Meeting Facilitated in partnership with This conference received financial support from: National Science Foundation Corteva Agriscience, Agriculture Division of DowDuPont Bayer BASF Syngenta NCGA KWS We thank these sponsors for their generosity! A special thank you for the in-kind support from the USDA-ARS. ii Table of Contents Cover Page ................................................................................................................... i Contributors ................................................................................................................. ii Table of Contents ......................................................................................................... iii General Information ..................................................................................................... iv From the Maize Genetics Cooperation............................................................................ vi Introducing the Committee on Outreach, Diversity, Inclusion & Education (CODIE)...viii Financial Aid Awards ......................................................................................................viii Data Management Made Simple...................................................................................... xi Program ........................................................................................................................ 1 List of Posters ............................................................................................................. -

Resolution of the Position of Restorer-Of-Fertility Gene
LOCALIZATION OF THE RF3 RESTORER-OF-FERTILITY GENE FOR MAIZE S-TYPE CYTOPLASMIC MALE STERILITY A Dissertation Presented to The Faculty of the Graduate School At the University of Missouri In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy By TIFFANY LANGEWISCH Dr. Kathleen Newton, Dissertation Supervisor December 2012 © Copyright by Tiffany Langewisch 2012 All Rights Reserved The undersigned, appointed by the dean of the Graduate School, have examined the Dissertation entitled LOCATION OF THE RF3 RESTORER-OF-FERTILITY GENE FOR MAIZE S-TYPE CYTOPLASMIC MALE STERILTY Presented by TIFFANY LANGEWISCH A candidate for the degree of Doctor of Philosophy And hereby certify that, in their opinion, it is worthy of acceptance. Kathleen Newton James Birchler Sherry Flint-Garcia Chris Pires ACKNOWLEDGEMENTS I would first like to acknowledge members of my committee, past and present, including Drs. Kathleen Newton, James Birchler, Sherry Flint-Garcia, Michael McMullen, Christopher Pires, and Karen Cone. My committee has provided helpful input and guidance throughout my dissertation research. I would like to thank them for their invaluable advice in all aspects of my graduate studies. As my advisor, Kathleen Newton has particularly provided advice, guidance, and support. This research would not have been possible without the development of Rf3 near-isogenic lines by Dr. Susan Gabay-Laughnan. She has been a crucial resource for seed and feedback throughout the years. Barbara Sonderman is thanked for her tireless effort of keeping my “babies” happy and healthy in the greenhouse. I thankfully acknowledge everyone in the Newton lab for discussions, field assistance, and help in my everyday research. -

Alternative Ac/Ds Transposition Induces Major Chromosomal Rearrangements in Maize
Downloaded from genesdev.cshlp.org on October 6, 2021 - Published by Cold Spring Harbor Laboratory Press Alternative Ac/Ds transposition induces major chromosomal rearrangements in maize Jianbo Zhang,1 Chuanhe Yu,1 Vinay Pulletikurti,2,4 Jonathan Lamb,3,5 Tatiana Danilova,3 David F. Weber,2 James Birchler,3 and Thomas Peterson1,6 1Department of Genetics, Development and Cell Biology, and Department of Agronomy, Iowa State University, Ames, Iowa 50011, USA; 2School of Biological Sciences, Illinois State University, Normal, Illinois 61790, USA; 3Division of Biological Sciences, University of Missouri, Columbia, Missouri 65211, USA Barbara McClintock reported that the Ac/Ds transposable element system can generate major chromosomal rearrangements (MCRs), but the underlying mechanism has not been determined. Here, we identified a series of chromosome rearrangements derived from maize lines containing pairs of closely linked Ac transposable element termini. Molecular and cytogenetic analyses showed that the MCRs in these lines comprised 17 reciprocal translocations and two large inversions. The breakpoints of all 19 MCRs are delineated by Ac termini and characteristic 8-base-pair target site duplications, indicating that the MCRs were generated by precise trans- position reactions involving the Ac termini of two closely linked elements. This alternative transposition mechanism may have contributed to chromosome evolution and may also occur during V(D)J recombination resulting in oncogenic translocations. [Keywords: V(D)J recombination; chromosome rearrangements; hAT elements; transposition] Supplemental material is available at http://www.genesdev.org. Received December 30, 2008; revised version accepted February 11, 2009. In the 1940s, Barbara McClintock reported that the maize aberrant Ds transposition that results in fusion of sister Activator (Ac) element could induce transposition of the chromatids, chromosome breakage, and formation of nonautonomous Dissociation (Ds) element, which she deletions has been described (English et al. -

Cell Size, Polyploidy, and Sex Determination in Nasonia
Cell size, polyploidy, and sex determination in Nasonia Supervision by: Kelley Leung Contact: [email protected], room 5172.0662; language: English olyploidy is the condition of having more Pthan the usual number of chromosome sets. In many ways polyploidy is detrimental because of problems of infertility and abnormal cell biol- ogy. However, polyploidization has happened many times via whole genome duplication in the evolutionary tree (including for humans!). It may have provided benefits such as addition- al gene copies and increased hardiness. This begs the question, how do polyploids overcome disadvantages to derive benefits? One possible adaptation that has allowed polyploids to persist is cell reduction mech- anisms. Polyploid cells are larger than normal cells. In vertebrate polyploids, cell re- duction mechanisms are used to retain fairly normal overall body size and physiology. However, it is unknown if this also holds for invertebrate polyploids, including insects. We will use the parasitoid wasp system Nasonia vitripennis to investigate whether there are cell reduction mechanisms in insect polyploids. Like all hymenopterans, N. vitripen- nis has a haplodiploid sex determination system: normally, unfertilized eggs become haploid (1n) males and fertilized eggs become diploid (2n) females. However, there are many ways that ploidy levels change. Some are naturally occurring. Others can be created by using RNAi knockdown to obtain null mutants of various sex determination genes. These lines produce haploid (1n) females, diploid males -

Multiple Large-Scale Gene and Genome Duplications During the Evolution of Hexapods
Multiple large-scale gene and genome duplications during the evolution of hexapods Zheng Lia,1, George P. Tileyb,c,1, Sally R. Galuskaa, Chris R. Reardona, Thomas I. Kiddera, Rebecca J. Rundella,d, and Michael S. Barkera,2 aDepartment of Ecology and Evolutionary Biology, University of Arizona, Tucson, AZ 85721; bDepartment of Biology, University of Florida, Gainesville, FL 32611; cDepartment of Biology, Duke University, Durham, NC 27708; and dDepartment of Environmental and Forest Biology, State University of New York College of Environmental Science and Forestry, Syracuse, NY 13210 Edited by Michael Freeling, University of California, Berkeley, CA, and approved March 12, 2018 (received for review June 14, 2017) Polyploidy or whole genome duplication (WGD) is a major contrib- than 800,000 described hexapod species (25) are known polyploids utor to genome evolution and diversity. Although polyploidy is (17, 20). However, until recently there were limited data available recognized as an important component of plant evolution, it is to search for evidence of paleopolyploidy among the hexapods generally considered to play a relatively minor role in animal and other animal clades. Thus, the contributions of polyploidy to evolution. Ancient polyploidy is found in the ancestry of some animal evolution and the differences with plant evolution have animals, especially fishes, but there is little evidence for ancient remained unclear. WGDs in other metazoan lineages. Here we use recently published To search for evidence of WGDs among the hexapods, we transcriptomes and genomes from more than 150 species across the leveraged recently released genomic data for the insects (26). insect phylogeny to investigate whether ancient WGDs occurred Combined with additional datasets from public databases, we assembled 128 transcriptomes and 27 genomes with at least one during the evolution of Hexapoda, the most diverse clade of animals. -

Curriculum Vitae
Curriculum Vitae Rick Masonbrink Home: 1222 Scholl Road, Ames, IA 50014 United States Office: 208 Bessey Hall, Ames, IA 50011 United States Home Phone and Work Phone: (660) 424-4118 Email: [email protected] Education BS Biology, Northwest Missouri State University (2006) Ph.D. Genetics, University of Missouri (2012) Grants NSF National Plant Genome Initiative Postdoctoral Research Fellowship ($207,000/3yrs) Research Experience 1/2006 - 5/2006 Undergraduate Researcher: Department of Biology, Northwest Missouri State University. Principal Investigator: Dr. Jeffrey Thornsberry Research: Genetic drift in freshwater mussels 8/2006 – 05/2012 Research Assistant: Department of Biology, University of Missouri. Principal Investigator: Dr. James Birchler Research: Investigating the effects of minichromosomes in maize 07/2012 – 06/2013 Postdoctoral Research Associate: Department of EEOB, Iowa State University. Principal Investigator: Dr. Jonathan Wendel Research: Centromere evolution and cyto-nuclear coevolution in Gossypium 07/2013 – present Postdoctoral Research Fellow: Department of EEOB, Iowa State University. Principal Investigator: Dr. Jonathan Wendel Research: Centromere evolution in Gossypium Mentoring Experience 06/2014 – 08/2014 Mentored a high school teacher in cytology 06/2013 – 08/2013 Mentored Justin Conover in bioinformatics 11/2012 – 05/2013 Mentored Josef Jareczek in molecular and phylogenetic techniques 02/2011 – 05/2012 Mentored and trained undergraduates in molecular and cytological techniques Teaching Experience 8/2006 - 12/2006 Biology 110 - Undergraduate level introductory biology, taught seminars and labs 1/2007 - 5/2007 General Genetics - Undergraduate level introductory genetics, taught seminars Skills Fluorescence in-situ hybridization, PCR, immunostaining, plant transformation and regeneration, chromatin immunoprecipitation, molecular cloning, QPCR, QRTPCR, proficient in Microsoft Office, bioinformatics, phylogenetics, UNIX, Perl, and Python programming, analysis of large-scale sequencing datasets Current Research 1. -

Swyersnathan.Pdf
DEVELOPMENT OF AN AMENABLE SYSTEM FOR SITE-SPECIFIC ADDITION TO A MAIZE CHROMOSOME _______________________________________ A Dissertation presented to the Faculty of the Graduate School at the University of Missouri-Columbia _______________________________________________________ In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy _____________________________________________________ by NATHAN CHARLES SWYERS Dr. James A. Birchler, Dissertation Supervisor December, 2019 The undersigned, appointed by the dean of the Graduate School, have examined the dissertation entitled DEVELOPMENT OF AN AMENABLE SYSTEM FOR SITE-SPECIFIC ADDITION TO A MAIZE CHROMOSOME Presented by Nathan Charles Swyers, A candidate for the degree of Doctor of Philosophy, And hereby certify that, in their opinion, it is worthy of acceptance. Dr. James A. Birchler Dr. Kathleen Newton Dr. David Braun Dr. Sherry Flint-Garcia ACKNOWLEDGMENTS The following list of people helped make this work possible: Birchler Lab: MU Biological Sciences: • Rebecca Ballew • Patrice Albert • David Braun • James A. Birchler • Lori Eggert • Weihong Chen • Nila Emerich • Robert Gaeta • Benjamin Julius • Rick Masonbrink • Melody Kroll • Ryan Donohue • Kathleen Newton • Ryan Douglas • Will Swatson • Lin Sun Family • Adam Johnson • Amie Swyers • Zhi Gao • Gideon Swyers • Nathaniel Graham • Michael Swyers • Becca Lukasak • Brenda Swyers • Morgan McCaw • Charles Swyers • Xiaowen Shi • Rita Henderson • Jon Cody • Michael Noble • Hua Yang • Jill Noble • Changzeng Zhao • Robert -

© American Society of Plant Biologists ADVANCING the SCIENCE of PLANT BIOLOGY This Article Is a Plant Cell Advance Online Publication
Centromere Pairing in Early Meiotic Prophase Requires Active Centromeres and Precedes Installation of the Synaptonemal Complex in Maize Jing Zhang, Wojciech P. Pawlowski and Fangpu Han Plant Cell; originally published online October 18, 2013; DOI 10.1105/tpc.113.117846 This information is current as of October 21, 2013 Supplemental Data http://www.plantcell.org/content/suppl/2013/10/11/tpc.113.117846.DC1.html Permissions https://www.copyright.com/ccc/openurl.do?sid=pd_hw1532298X&issn=1532298X&WT.mc_id=pd_hw1532298X eTOCs Sign up for eTOCs at: http://www.plantcell.org/cgi/alerts/ctmain CiteTrack Alerts Sign up for CiteTrack Alerts at: http://www.plantcell.org/cgi/alerts/ctmain Subscription Information Subscription Information for The Plant Cell and Plant Physiology is available at: http://www.aspb.org/publications/subscriptions.cfm © American Society of Plant Biologists ADVANCING THE SCIENCE OF PLANT BIOLOGY This article is a Plant Cell Advance Online Publication. The date of its first appearance online is the official date of publication. The article has been edited and the authors have corrected proofs, but minor changes could be made before the final version is published. Posting this version online reduces the time to publication by several weeks. Centromere Pairing in Early Meiotic Prophase Requires Active Centromeres and Precedes Installation of the Synaptonemal Complex in MaizeW Jing Zhang,a,b Wojciech P. Pawlowski,c and Fangpu Hana,1 a State Key Laboratory of Plant Cell and Chromosome Engineering, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing 100101, China b University of the Chinese Academy of Sciences, Beijing 100049, China c Department of Plant Breeding and Genetics, Cornell University, Ithaca, New York 14853 ORCID ID: 0000-0001-8393-3575 (F.H.). -

An Interview with M. Gerald Neuffer M. Gerald “Gerry”
Maize Genetics Cooperation Newsletter vol 88 2014 M Gerald Neuffer Interview Page 1 of 17 An Interview with M. Gerald Neuffer M. Gerald “Gerry” Neuffer received his bachelor's degree in agronomy from the University of Idaho in 1947 and the doctorate degree in field crops from the University of Missouri in 1952 under the mentorship of Lewis J. Stadler. Following a short postdoctoral position at the University of Missouri, under both Stadler and John Laughnan, Neuffer was appointed assistant professor in the Department of Field Crops at the University of Missouri in 1951, and after Stadler’s death in 1954, in 1955 assumed the university position previously held by Stadler. He was tenured and promoted to associate professor in 1956 and full professor in 1966. He chaired the Department of Genetics from 1967 to 1969. He retired from the Department of Agronomy (now, Division of Plant Sciences) in 1992, and he currently holds the title of professor emeritus in the Division of Plant Sciences. Neuffer has had a tremendous influence on the history of maize genetics over the last half century. His early research has contributed to our understanding of the compound nature of the R1 locus, the characteristics of the compound A1 locus and its response to the Dt transposon system, the discovery of the aleurone and plant, color factor bz2, tetrasporic embryo sac development, the paucity of auxotrophs (Sheridan and Chang, 1994). He is credited for developing, with his long-term colleague Edward H. Coe, the paraffin oil method for treating corn pollen with ethyl methanesulfonate (EMS) and nitrosoguanidine (NG) as well as for the use of chromosome breaking Ds method to study chromosome structure and gene function. -

The Frequency of Polyploid Speciation in Vascular Plants
The frequency of polyploid speciation in vascular plants Troy E. Wooda,b,1, Naoki Takebayashic, Michael S. Barkerb,d, Itay Mayrosee, Philip B. Greenspoond, and Loren H. Riesebergb,d aInstitute for Evolution and Biodiversity, University of Mu¨nster, 48149 Mu¨nster, Germany; bDepartment of Biology, Indiana University, Bloomington, IN 47405; cInstitute of Arctic Biology and Department of Biology and Wildlife, University of Alaska, Fairbanks, AK 99775; and Departments of dBotany and eZoology, University of British Columbia, Vancouver, BC, Canada V6T 1Z4 Edited by Peter R. Crane, University of Chicago, Chicago, IL, and approved June 23, 2009 (received for review November 13, 2008) Since its discovery in 1907, polyploidy has been recognized as an contrast, polyploid incidence is distributed less equitably among 2 ϭ Ͻ 2 ϭ important phenomenon in vascular plants, and several lines of families ( 198 4,259.41, P 0.00001, R 0.116). Overall, with evidence indicate that most, if not all, plant species ultimately have the exception of the species-poor gymnosperms, vascular plant a polyploid ancestry. However, previous estimates of the fre- species derived from recent polyploid events are ubiquitous and quency of polyploid speciation suggest that the formation and represent a large fraction of named diversity, a pattern that also establishment of neopolyploid species is rare. By combining infor- holds for bryophytes (12). Interestingly, generic base counts mation from the botanical community’s vast cytogenetic and are negatively associated with polyploid incidence in angio- 2 ϭ Ͻ 2 ϭ phylogenetic databases, we establish that 15% of angiosperm and sperms ( 3 2,798.01, P 0.00001, R 0.085; Fig.