Parturition Time for the Blacktip Shark, Carcharhinus Limbatus (Carcharhiniformes: Carcharhinidae), in Southwestern Atlantic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NOAA Technical Memorandum NMFS-SEFSC-626
NOAA Technical Memorandum NMFS-SEFSC-626 RELATIVE ABUNDANCE OF SMALLTOOTH SAWFISH (Pristis pectinata) BASED ON THE EVERGLADES NATIONAL PARK CREEL SURVEY BY JOHN K. CARLSON and JASON OSBORNE U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Marine Fisheries Service Southeast Fisheries Science Center Panama City Laboratory 3500 Delwood Beach Rd. Panama City, FL 32408 February 2012 NOAA Technical Memorandum NMFS-SEFSC-626 RELATIVE ABUNDANCE OF SMALLTOOTH SAWFISH (Pristis pectinata) BASED ON THE EVERGLADES NATIONAL PARK CREEL SURVEY BY JOHN K. CARLSONa, and JASON OSBORNEb aNational Marine Fisheries Service Southeast Fisheries Science Center 3500 Delwood Beach Road Panama City, FL 32408 bNational Park Service South Florida Natural Resource Center 40001 State Road 9336 Homestead, FL, 33034 U. S. DEPARTMENT OF COMMERCE Rebecca M. Blank, Acting Secretary NATIONAL OCEANIC AND ATMOSPHERIC ADMINISTRATION Jane Lubchenco, Under Secretary for Oceans and Atmosphere NATIONAL MARINE FISHERIES SERVICE Eric Schwaab, Assistant Administrator for Fisheries February 2012 This Technical Memorandum series is used for documentation and timely communication of preliminary results, interim reports, or similar special-purpose information. Although the memoranda are not subject to complete formal review, editorial control, or detailed editing, they are expected to reflect sound professional work. NOTICE The National Marine Fisheries Service (NMFS) does not approve, recommend or endorse any proprietary product or material mentioned in this publication. No reference shall be made to NMFS or to this publication furnished by NMFS in any advertising or sales promotion which would imply that NMFS approves, recommends, or endorses any proprietary product or proprietary material mentioned herein which has as its purpose any intent to cause directly or indirectly the advertised product to be used or purchased because of this NMFS publication. -

Spinner Shark, Carcharhinus Brevipinna
Published Date: 1 March 2019 Spinner Shark, Carcharhinus brevipinna Report Card Sustainable assessment IUCN Red List IUCN Red List Australian Least Concern Global Near Threatened Assessment Assessment Assessors Burgess, G.H. & Smart, J.J. Report Card Remarks In Australia, fishing pressure is currently well managed Summary The Spinner Shark is a common, coastal pelagic shark found in warm- temperate and tropical waters across the globe. It frequents nearshore waters and is often captured in commercial and recreational fisheries. It is sensitive to fishing pressure and habitat degradation of coastal nursery Source: CSIRO national Fish Collection. License: CC By Attribution. habitats. Little information is known on the status of Spinner Sharks throughout its distribution. Within Australia, fishing pressure is currently well managed. The Spinner Shark is assessed globally as Near Threatened (IUCN) and in Australia as Least Concern (IUCN), while Australian stocks are classified as Sustainable (SAFS). Distribution Within Australia, the Spinner Shark is found across northern Australia, from Walpole (Western Australia), throughout the Northern Territory, Queensland and to southern New South Wales (Last and Stevens 2009). It is distributed throughout the world, including the east coast of the United States, Brazil, Mediterranean Sea, west coast of Central Africa, South Africa, Madagascar and throughout the Indo-Pacific. A recent genetic study detected evidence suggesting there may be multiple, genetically- distinct stocks throughout its Australian range (Geraghty et al. 2013). Stock structure and status There is currently very little information on population size and stock status for the Spinner Shark in Australian waters. A suite of management measures introduced from 2009 have led to a substantial reduction in fishing effort targeting adults in New South Wales waters. -
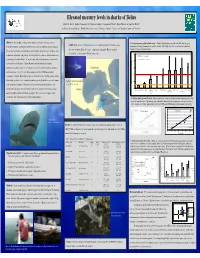
Mercury in Sharks of Belize, 2008
Elevated mercury levels in sharks of Belize David C. Evers 1, Rachel T. Graham 2, Neil Hammerschlag 3, Christopher Perkins 4, Robert Michener 5, and Tim Divoll 1 BioDiversity Research Institute 1, Wildlife Conservation Society 2, University of Miami 3, University of Connecticut 4, and Boston University 5. Abstract: Mercury (Hg) loading in global aquatic ecosystems is a growing concern. 1. Mercury exposure profile by shark species - Highest Hg levels were recorded in the bull, blacktip, great Study Area : A total of 14 distinct locations were sampled in the Gulf of Honduras along Compelling evidence of widespread adverse effects in fish and wildlife populations indicates hammerhead, scalloped hammerhead, and nurse sharks. Lowest Hg levels were recorded in the bonnethead, the coast of southern Belize (red circle). Comparison shark muscle Hg concentrations sharpnose, lemon and sandbar sharks. the rate of transformation to methylmercury is problematic. Long-lived, apex predators such from the U.S. are from southern Florida (white circle). 2.5 as sharks are at high risk to Hg toxicity. We investigated the occurrence of Hg in sharks from USEPA Advisory = 0.3 ug/g, ww coastal waters of southern Belize. In our pilot study, 101 sharks representing 9 species were FDA & WHO Advisory = 1.0 ug/g, ww 2.0 analyzed for muscle Hg levels. Highest Hg levels were recorded in bull, blacktip, hammerhead, and nurse sharks. Lowest Hg levels were recorded in bonnethead, sharpnose, 1.5 and lemon sharks. Over 88% of the sharks sampled exceeded USEPA human health 1.0 consumption standards. Muscle Hg strongly correlated with size for blacktip and nurse sharks. -

An Overview of Shark Utilisation in the Coral Triangle Region (PDF, 550
WORKING TOGETHER FOR SUSTAINABLE SHARK FISHERIES AN OVERVIEW OF SHARK UTILISATION IN THE CORAL TRIANGLE REGION Written by Mary Lack Director, Shellack Pty Ltd Glenn Sant Fisheries Programme Leader, TRAFFIC & Senior Fellow, ANCORS Published in September 2012 This report can be downloaded from wwf.panda.org/coraltriangle Citation Lack M. and Sant G. (2012). An overview of shark utilisation in the Coral Triangle region. TRAFFIC &WWF. Photo cover © naturepl.com / Jeff Rotman / WWF-Canon Thanks to the Rufford Lang Foundation for supporting the development of this publication 2 An Overview Of Shark Utilisation In The Coral Triangle Region ACRONYMS ASEAN Association of Southeast Asian Nations BFAR Bureau of Fisheries and Aquatic Resources (the Philippines) CCSBT Commission for the Conservation of Southern Bluefin Tuna CITES Convention on International Trade in Endangered Species of Wild Fauna and Flora CMM Conservation and Management Measure CMS Convention on Migratory Species of Wild Animals CNP Co-operating Non-Contracting party COFI Committee on Fisheries (of FAO) CoP Conference of the Parties (to CITES) EEZ Exclusive Economic Zone EU European Union FAO Food and Agriculture Organization of the United Nations IOTC Indian Ocean Tuna Commission IPOA-Sharks International Plan of Action for the Conservation and Management of Sharks IUU Illegal, Unreported and Unregulated (fishing) MoU Memorandum of Understanding on the Conservation of Migratory Sharks (CMS) nei Not elsewhere included NPOA-Sharks National Plan of Action for the Conservation and -

Feeding Habits of Blacktip Sharks, Carcharhinus Limbatus, and Atlantic
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2002 Feeding habits of blacktip sharks, Carcharhinus limbatus, and Atlantic sharpnose sharks, Rhizoprionodon terraenovae, in Louisiana coastal waters Kevin Patrick Barry Louisiana State University and Agricultural and Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Part of the Oceanography and Atmospheric Sciences and Meteorology Commons Recommended Citation Barry, Kevin Patrick, "Feeding habits of blacktip sharks, Carcharhinus limbatus, and Atlantic sharpnose sharks, Rhizoprionodon terraenovae, in Louisiana coastal waters" (2002). LSU Master's Theses. 66. https://digitalcommons.lsu.edu/gradschool_theses/66 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. FEEDING HABITS OF BLACKTIP SHARKS, CARCHARHINUS LIMBATUS, AND ATLANTIC SHARPNOSE SHARKS, RHIZOPRIONODON TERRAENOVAE, IN LOUISIANA COASTAL WATERS A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College In partial fulfillment of the Requirements for the degree of Master of Science in The Department of Oceanography and Coastal Sciences by Kevin P. Barry B.S., University of South Alabama, 1996 August 2002 AKNOWLEDGEMENTS I would like to first and foremost thank my major professor, Dr. Richard Condrey, for giving me the opportunity to pursue this graduate degree. His willingness to accompany me during sampling trips, his enthusiasm and interest in my research topic, and his guidance throughout my time here has forged more than a major professor/graduate student relationship; it has formed a friendship as well. -

Survival of the Blacktip Shark, Carcharhinus Limbatus
University of Rhode Island DigitalCommons@URI Open Access Master's Theses 2013 Survival of the blacktip shark, Carcharhinus limbatus William A. Swinsburg University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/theses Recommended Citation Swinsburg, William A., "Survival of the blacktip shark, Carcharhinus limbatus" (2013). Open Access Master's Theses. Paper 14. https://digitalcommons.uri.edu/theses/14 This Thesis is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Master's Theses by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. SURVIVAL OF THE BLACKTIP SHARK, CARCHARHINUS LIMBATUS BY WILLIAM A. SWINSBURG A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN BIOLOGICAL AND ENVIRONMENTAL SCIENCES UNIVERSITY OF RHODE ISLAND 2013 MASTER OF BIOLOGICAL AND ENVIRONMENTAL SCIENCES THESIS OF WILLIAM A. SWINSBURG APPROVED: Thesis Committee: Major Professor David Bengtson Nancy Kohler Jeremy Collie Graham Forrester Nasser H. Zawia DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2013 ABSTRACT Proportional survival (S) is a crucial life-history parameter in population dynamics, natural selection, and management of harvested stocks; variations in survival due to age, sex, or geographic region may have large effects on the success of managing fish stocks. The blacktip shark, Carcharhinus limbatus, is the most abundantly harvested shark species in American fisheries. Direct estimates of survival are preferred, but all current survival estimates for this species are either focused on young-of-the-year (YOY) or based on indirect methods. -

Common Sharks of the Northern Gulf of Mexico So You Caught a Sand Shark?
Common Sharks of the Northern Gulf of Mexico So you caught a sand shark? Estuaries are ecosystems where fresh and saltwater meet The northern Gulf of Mexico is home to several shark and mix. Estuaries provide nursery grounds for a wide species. A few of these species very closely resemble variety of invertebrate species such as oysters, shrimp, one another and are commonly referred to as and blue crabs along finfishes including croaker, red “sand sharks.” drum, spotted seatrout, tarpon, menhaden, flounder and many others. This infographic will help you quickly differentiate between the different “sand sharks” and also help you Because of this abundance, larger animals patrol coastal identify a few common offshore species. Gulf waters for food. Among these predators are a number of shark species. Sharpnose (3.5 ft) Blacknose (4ft) Finetooth (4ft) Blacktip (5ft) Maximum size of Human (avg. 5.5ft) . the coastal Spinner (6ft) sharks are Bull (8ft) depicted in scale Silky (9ft) Scalloped Hammerhead (10ft) Great Hammerhead (13ft) Maximum Adult Size Adult Maximum Tiger (15ft) 5 ft 10 ft 15 ft Blacktip shark Atlantic sharpnose shark Carcharhinus limbatus Rhizoprionodon terraenovae Spinner shark Easy ID: White “freckles” on the body Easy ID: Pointed snout, anal fin lacks a black tip Carcharhinus brevipinna Finetooth shark Blacknose shark Carcharhinus isodon Carcharhinus acronotus Easy ID: Black tip on anal fin present Easy ID: Distinct lack of black markings on fins, extremely pointed snout Easy ID: Distinct black smudge on the tip of the snout, -

Blacktip Reef Sharks, Carcharhinus Melanopterus, Have High Genetic Structure and Varying Demographic Histories in Their Indopaci
Molecular Ecology (2014) 23, 5193–5207 doi: 10.1111/mec.12936 Blacktip reef sharks, Carcharhinus melanopterus, have high genetic structure and varying demographic histories in their Indo-Pacific range THOMAS M. VIGNAUD,* JOHANN MOURIER,* JEFFREY A. MAYNARD,*† RAPHAEL LEBLOIS,‡ JULIA SPAET,§ ERIC CLUA,¶ VALENTINA NEGLIA* and SERGE PLANES* *Laboratoire d’Excellence “CORAIL”, USR 3278 CNRS – EPHE, CRIOBE, BP 1013 - 98 729 Papetoai, Moorea, Polynesie, Franßcaise, †Department of Ecology and Evolutionary Biology, Cornell University, E241 Corson Hall, Ithaca, NY 14853, USA, ‡INRA, UMR1062 CBGP, F-34988 Montferrier-sur-Lez, France, §Red Sea Research Center, King Abdullah University of Science and Technology, 23955-6900 Thuwal, Saudi Arabia, ¶French Ministry of Agriculture and Fisheries, 75007 Paris, France Abstract For free-swimming marine species like sharks, only population genetics and demo- graphic history analyses can be used to assess population health/status as baseline population numbers are usually unknown. We investigated the population genetics of blacktip reef sharks, Carcharhinus melanopterus; one of the most abundant reef-associ- ated sharks and the apex predator of many shallow water reefs of the Indian and Pacific Oceans. Our sampling includes 4 widely separated locations in the Indo-Pacific and 11 islands in French Polynesia with different levels of coastal development. Four- teen microsatellite loci were analysed for samples from all locations and two mito- chondrial DNA fragments, the control region and cytochrome b, were examined for 10 locations. For microsatellites, genetic diversity is higher for the locations in the large open systems of the Red Sea and Australia than for the fragmented habitat of the smaller islands of French Polynesia. -

First Photographic Inland Record of Blacktip Reef Sharks Carcharhinus Melanopterus (Carcharhiniformes: Carcharhinidae) in Indonesian Waters
Ecologica Montenegrina 24: 6-10 (2019) This journal is available online at: www.biotaxa.org/em First photographic inland record of blacktip reef sharks Carcharhinus melanopterus (Carcharhiniformes: Carcharhinidae) in Indonesian waters MUHAMMAD IQBAL1, RIO FIRMAN SAPUTRA2, ARUM SETIAWAN3 & INDRA YUSTIAN3* 1Biology Program, Faculty of Science, Sriwijaya University, Jalan Padang Selasa 524, Palembang, Sumatera Selatan 30129, Indonesia. 2Conservation Biology Program, Faculty of Science, Sriwijaya University, Jalan Padang Selasa 524, Palembang, Sumatera Selatan 30129, Indonesia. 3Department of Biology, Faculty of Science, Sriwijaya University, Jalan Raya Palembang-Prabumulih km 32, Indralaya, Sumatera Selatan 30662, Indonesia. * Corresponding author: E-mail: [email protected] Received 2 October 2019 │ Accepted by V. Pešić: 1 November 2019 │ Published online 7 November 2019. Abstract Blacktip reef sharks Carcharhinus melanopterus were caught and photographed by local people on 6 March 2019 during a flash flood in Sentani, Jayapura district, Papua province, Indonesia. The presence of C. melanopterus in Sentani represents the first inland record (c. 20 km) for this species in Indonesia. All individuals found were small-sized sharks (c. 500-600 mm), suggesting very young juveniles. We presume their occurence in Sentani a few hours after a flash flood is in response to seasonal freshwater inflow to estuarine environment. Key words: distribution, whaler shark, Carcharhinus melanopterus, Indonesia, Papua, freshwater. Introduction Elasmobranch fishes evolved as a group in the marine environment during the Devonian period, and those presently living in freshwater presumably represent a more recent adaption (Perlman & Goldstein 1988). The blacktip reef shark Carcharhinus melanopterus (Quoy & Gaimard, 1824) is a medium-sized elasmobranch of whaler shark (family Carcharhinidae) that reportedly grows up to 1.5 m (total length) and occurs throughout the tropics from the Red and Indian Sea (Chin et al. -

Mississippi's Sharks and Rays an Educational Guide for Mississippi
Mississippi’s Sharks and Rays An educational guide for Mississippi Aquarium Photo provided by Mississippi Aquarium Mississippi’s Sharks and Rays An educational guide for Mississippi Aquarium Edited by Marcus Drymon, PhD1,2 Illustrations by Bryan Huerta-Beltran1 Species data compiled by Matthew Jargowsky1,2 and Emily Seubert1 1Mississippi State University Extension Service 2Mississippi-Alabama Sea Grant Consortium MASGP-21-016 Contents 2 Using This Guide ............................3 Mississippi Hammerheads Bonnethead ............................ 24 Anatomy of a Shark .........................4 Scalloped hammerhead .............. 26 Anatomy of a Ray ...........................5 Great hammerhead ................... 28 Mississippi Aquarium Sharks Mississippi Deepwater Sharks Nurse shark ..............................6 Gulper shark ........................... 30 Sandbar shark ...........................8 Sharpnose sevengill shark ........... 32 Sand tiger shark ....................... 10 Goblin shark ........................... 34 Common Mississippi Sharks Mississippi Aquarium Rays Atlantic sharpnose shark ............. 12 Cownose ray ........................... 36 Blacknose shark ....................... 14 Atlantic stingray ...................... 38 Blacktip shark ......................... 16 Southern stingray ..................... 40 Mississippi Apex Predators Other Mississippi Rays Bull shark .............................. 18 Bluntnose stingray .................... 42 Tiger shark ..................................20 Smooth butterfly ray -

Common Blacktip Shark (Australia-New Guinea Subpopulation), Carcharhinus Limbatus
Published Date: 1 March 2019 Common Blacktip Shark (Australia-New Guinea subpopulation), Carcharhinus limbatus Report Card Sustainable assessment IUCN Red List IUCN Red List Australian Least Concern Global Near Threatened Assessment Assessment Assessors Burgess, G.H., Branstetter, S. & Chin, A. In Australia, catch levels strictly managed and deemed sustainable. This Report Card Remarks species has been assessed in the Status of Australian Fish Stocks Reports http://www.fish.gov.au/ Summary The Common Blacktip Shark (Australia- New Guinea subpopulation) is a medium-sized shark common in warm temperate and tropical waters throughout northern Australia and Papua New Guinea. It frequents inshore waters as adults and has coastal nursery habitats, making it subject to fishing pressure and habitat degradation. In Australian waters it is regularly encountered in recreational and commercial fisheries. Within Papua New Source: Albert Kok/Wikimedia Commons. License: Public Domain Guinea, it has likely been subject to substantial fishing pressure. In Australia, fisheries and harvest levels are carefully managed and are deemed sustainable. Therefore, the Common Blacktip Shark (Australia-New Guinea subpopulation) is assessed as Near Threatened (IUCN) and in Australia as Least Concern (IUCN), while Australian stocks are classified as Sustainable (SAFS). Distribution The Common Blacktip Shark occurs globally in tropical and subtropical waters. Within Australia, it is found from Cape Naturaliste (Western Australia), through the Northern Territory and Queensland to southern New South Wales (Last and Stevens 2009). Stock structure and status In Australian waters, the Common Blacktip Shark is comprised of three genetically discrete stocks: Western Australia/Northern Territory stock, Gulf of Carpentaria Stock and a Gulf of Carpentaria/Queensland/New South Wales stock (Grubert et al. -

The Conservation Status of Pelagic Sharks and Rays
The Conservation Status of The Conservation Status of Pelagic Sharks and Rays The Conservation Status of Pelagic Sharks and Rays Pelagic Sharks and Rays Report of the IUCN Shark Specialist Group Pelagic Shark Red List Workshop Report of the IUCN Shark Specialist Group Tubney House, University of Oxford, UK, 19–23 February 2007 Pelagic Shark Red List Workshop Compiled and edited by Tubney House, University of Oxford, UK, 19–23 February 2007 Merry D. Camhi, Sarah V. Valenti, Sonja V. Fordham, Sarah L. Fowler and Claudine Gibson Executive Summary This report describes the results of a thematic Red List Workshop held at the University of Oxford’s Wildlife Conservation Research Unit, UK, in 2007, and incorporates seven years (2000–2007) of effort by a large group of Shark Specialist Group members and other experts to evaluate the conservation status of the world’s pelagic sharks and rays. It is a contribution towards the IUCN Species Survival Commission’s Shark Specialist Group’s “Global Shark Red List Assessment.” The Red List assessments of 64 pelagic elasmobranch species are presented, along with an overview of the fisheries, use, trade, and management affecting their conservation. Pelagic sharks and rays are a relatively small group, representing only about 6% (64 species) of the world’s total chondrichthyan fish species. These include both oceanic and semipelagic species of sharks and rays in all major and Claudine Gibson L. Fowler Sarah Fordham, Sonja V. Valenti, V. Camhi, Sarah Merry D. Compiled and edited by oceans of the world. No chimaeras are known to be pelagic. Experts at the workshop used established criteria and all available information to update and complete global and regional species-specific Red List assessments following IUCN protocols.