Blacktip Reef Sharks, Carcharhinus Melanopterus, Have High Genetic Structure and Varying Demographic Histories in Their Indopaci
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NOAA Technical Memorandum NMFS-SEFSC-626
NOAA Technical Memorandum NMFS-SEFSC-626 RELATIVE ABUNDANCE OF SMALLTOOTH SAWFISH (Pristis pectinata) BASED ON THE EVERGLADES NATIONAL PARK CREEL SURVEY BY JOHN K. CARLSON and JASON OSBORNE U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Marine Fisheries Service Southeast Fisheries Science Center Panama City Laboratory 3500 Delwood Beach Rd. Panama City, FL 32408 February 2012 NOAA Technical Memorandum NMFS-SEFSC-626 RELATIVE ABUNDANCE OF SMALLTOOTH SAWFISH (Pristis pectinata) BASED ON THE EVERGLADES NATIONAL PARK CREEL SURVEY BY JOHN K. CARLSONa, and JASON OSBORNEb aNational Marine Fisheries Service Southeast Fisheries Science Center 3500 Delwood Beach Road Panama City, FL 32408 bNational Park Service South Florida Natural Resource Center 40001 State Road 9336 Homestead, FL, 33034 U. S. DEPARTMENT OF COMMERCE Rebecca M. Blank, Acting Secretary NATIONAL OCEANIC AND ATMOSPHERIC ADMINISTRATION Jane Lubchenco, Under Secretary for Oceans and Atmosphere NATIONAL MARINE FISHERIES SERVICE Eric Schwaab, Assistant Administrator for Fisheries February 2012 This Technical Memorandum series is used for documentation and timely communication of preliminary results, interim reports, or similar special-purpose information. Although the memoranda are not subject to complete formal review, editorial control, or detailed editing, they are expected to reflect sound professional work. NOTICE The National Marine Fisheries Service (NMFS) does not approve, recommend or endorse any proprietary product or material mentioned in this publication. No reference shall be made to NMFS or to this publication furnished by NMFS in any advertising or sales promotion which would imply that NMFS approves, recommends, or endorses any proprietary product or proprietary material mentioned herein which has as its purpose any intent to cause directly or indirectly the advertised product to be used or purchased because of this NMFS publication. -

Sea Snakes You Can Easily Change the Color Theme of Your Poster by Going to the Presentation Poster
(—THIS SIDEBAR DOES NOT PRINT—) QUICK START (cont.) DESIGN GUIDE How to change the template color theme This PowerPoint 2007 template produces a 36”x48” Sea Snakes You can easily change the color theme of your poster by going to the presentation poster. You can use it to create your research DESIGN menu, click on COLORS, and choose the color theme of your choice. You can also create your own color theme. poster and save valuable time placing titles, subtitles, text, and graphics. Howard Moon We provide a series of online tutorials that will guide you through the poster design process and answer your poster production questions. To view our template tutorials, go Abstract Venom Reproduction Diet You can also manually change the color of your background by going to online to PosterPresentations.com and click on HELP DESK. VIEW > SLIDE MASTER. After you finish working on the master be sure to Sea Snakes (also known as Hydrophiinae) are reptiles that Since sea snakes come from Elapidae family, the majority of Sea snakes are ovoviviparous, except for laticauda, which is Sea snakes are carnivores that feed on fish, fish eggs, go to VIEW > NORMAL to continue working on your poster. When you are ready to print your poster, go online to inhabit in marine environments that are considered one of the the Hydrophiinae species possess venom glands. Species oviparous. Although sea snakes are air-breathing species, they mollusks, eels, etc. They usually wander around the coral reefs How to add Text PosterPresentations.com most aquatic vertebrates. These guys are found in warm such as beaked sea snake (Enhydrina schistose) can kill about mate in water. -

Hemiscyllium Ocellatum), with Emphasis on Branchial Circulation Kåre-Olav Stensløkken*,1, Lena Sundin2, Gillian M
The Journal of Experimental Biology 207, 4451-4461 4451 Published by The Company of Biologists 2004 doi:10.1242/jeb.01291 Adenosinergic and cholinergic control mechanisms during hypoxia in the epaulette shark (Hemiscyllium ocellatum), with emphasis on branchial circulation Kåre-Olav Stensløkken*,1, Lena Sundin2, Gillian M. C. Renshaw3 and Göran E. Nilsson1 1Physiology Programme, Department of Molecular Biosciences, University of Oslo, PO Box 1041, NO-0316 Oslo Norway and 2Department of Zoophysiology, Göteborg University, SE-405 30 Göteborg, Sweden and 3Hypoxia and Ischemia Research Unit, School of Physiotherapy and Exercise Science, Griffith University, PMB 50 Gold coast Mail Centre, Queensland, 9726 Australia *Author for correspondence (e-mail: [email protected]) Accepted 17 September 2004 Summary Coral reef platforms may become hypoxic at night flow in the longitudinal vessels during hypoxia. In the during low tide. One animal in that habitat, the epaulette second part of the study, we examined the cholinergic shark (Hemiscyllium ocellatum), survives hours of severe influence on the cardiovascular circulation during severe hypoxia and at least one hour of anoxia. Here, we examine hypoxia (<0.3·mg·l–1) using antagonists against muscarinic the branchial effects of severe hypoxia (<0.3·mg·oxygen·l–1 (atropine 2·mg·kg–1) and nicotinic (tubocurarine for 20·min in anaesthetized epaulette shark), by measuring 5·mg·kg–1) receptors. Injection of acetylcholine (ACh; –1 ventral and dorsal aortic blood pressure (PVA and PDA), 1·µmol·kg ) into the ventral aorta caused a marked fall in heart rate (fH), and observing gill microcirculation using fH, a large increase in PVA, but small changes in PDA epi-illumination microscopy. -

Assessing Interacting Global Change Stressors Via Experimental Studies on Sharks
W&M ScholarWorks VIMS Articles Virginia Institute of Marine Science 10-16-2020 The power struggle: assessing interacting global change stressors via experimental studies on sharks Ian A. Bouyoucos Sue-Ann Watson Serge Planes Colin A. Simpfendorfer Gail D. Schwieterman Virginia Institute of Marine Science See next page for additional authors Follow this and additional works at: https://scholarworks.wm.edu/vimsarticles Part of the Aquaculture and Fisheries Commons, and the Marine Biology Commons Recommended Citation Bouyoucos, Ian A.; Watson, Sue-Ann; Planes, Serge; Colin A. Simpfendorfer; Schwieterman, Gail D.; and et al, The power struggle: assessing interacting global change stressors via experimental studies on sharks (2020). Scientific Reports, 10, 19887. doi: 10.1038/s41598-020-76966-7 This Article is brought to you for free and open access by the Virginia Institute of Marine Science at W&M ScholarWorks. It has been accepted for inclusion in VIMS Articles by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. Authors Ian A. Bouyoucos, Sue-Ann Watson, Serge Planes, Colin A. Simpfendorfer, Gail D. Schwieterman, and et al This article is available at W&M ScholarWorks: https://scholarworks.wm.edu/vimsarticles/2009 www.nature.com/scientificreports OPEN The power struggle: assessing interacting global change stressors via experimental studies on sharks Ian A. Bouyoucos1,2*, Sue‑Ann Watson1,3, Serge Planes2,4, Colin A. Simpfendorfer5, Gail D. Schwieterman6, Nicholas M. Whitney7 & Jodie L. Rummer1 Ocean warming and acidifcation act concurrently on marine ectotherms with the potential for detrimental, synergistic efects; yet, efects of these stressors remain understudied in large predatory fshes, including sharks. -

Marine Reptiles
Species group report card – marine reptiles Supporting the marine bioregional plan for the North Marine Region prepared under the Environment Protection and Biodiversity Conservation Act 1999 Disclaimer © Commonwealth of Australia 2012 This work is copyright. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission from the Commonwealth. Requests and enquiries concerning reproduction and rights should be addressed to Department of Sustainability, Environment, Water, Population and Communities, Public Affairs, GPO Box 787 Canberra ACT 2601 or email [email protected] Images: A gorgonian wtih polyps extended – Geoscience Australia, Hawksbill Turtle – Paradise Ink, Crested Tern fishing – R.Freeman, Hard corals – A.Heyward and M.Rees, Morning Light – I.Kiessling, Soft corals – A.Heyward and M.Rees, Snubfin Dolphin – D.Thiele, Shrimp, scampi and brittlestars – A.Heyward and M.Rees, Freshwater sawfish – R.Pillans, CSIRO Marine and Atmospheric Research, Yellowstripe Snapper – Robert Thorn and DSEWPaC ii | Supporting the marine bioregional plan for the North Marine Region | Species group report card – marine reptiles CONTENTS Species group report card – marine reptiles ..........................................................................1 1. Marine reptiles of the North Marine Region .............................................................................3 2. Vulnerabilities and pressures ................................................................................................ -

Reef Fishes of the Bird's Head Peninsula, West
Check List 5(3): 587–628, 2009. ISSN: 1809-127X LISTS OF SPECIES Reef fishes of the Bird’s Head Peninsula, West Papua, Indonesia Gerald R. Allen 1 Mark V. Erdmann 2 1 Department of Aquatic Zoology, Western Australian Museum. Locked Bag 49, Welshpool DC, Perth, Western Australia 6986. E-mail: [email protected] 2 Conservation International Indonesia Marine Program. Jl. Dr. Muwardi No. 17, Renon, Denpasar 80235 Indonesia. Abstract A checklist of shallow (to 60 m depth) reef fishes is provided for the Bird’s Head Peninsula region of West Papua, Indonesia. The area, which occupies the extreme western end of New Guinea, contains the world’s most diverse assemblage of coral reef fishes. The current checklist, which includes both historical records and recent survey results, includes 1,511 species in 451 genera and 111 families. Respective species totals for the three main coral reef areas – Raja Ampat Islands, Fakfak-Kaimana coast, and Cenderawasih Bay – are 1320, 995, and 877. In addition to its extraordinary species diversity, the region exhibits a remarkable level of endemism considering its relatively small area. A total of 26 species in 14 families are currently considered to be confined to the region. Introduction and finally a complex geologic past highlighted The region consisting of eastern Indonesia, East by shifting island arcs, oceanic plate collisions, Timor, Sabah, Philippines, Papua New Guinea, and widely fluctuating sea levels (Polhemus and the Solomon Islands is the global centre of 2007). reef fish diversity (Allen 2008). Approximately 2,460 species or 60 percent of the entire reef fish The Bird’s Head Peninsula and surrounding fauna of the Indo-West Pacific inhabits this waters has attracted the attention of naturalists and region, which is commonly referred to as the scientists ever since it was first visited by Coral Triangle (CT). -

Spinner Shark, Carcharhinus Brevipinna
Published Date: 1 March 2019 Spinner Shark, Carcharhinus brevipinna Report Card Sustainable assessment IUCN Red List IUCN Red List Australian Least Concern Global Near Threatened Assessment Assessment Assessors Burgess, G.H. & Smart, J.J. Report Card Remarks In Australia, fishing pressure is currently well managed Summary The Spinner Shark is a common, coastal pelagic shark found in warm- temperate and tropical waters across the globe. It frequents nearshore waters and is often captured in commercial and recreational fisheries. It is sensitive to fishing pressure and habitat degradation of coastal nursery Source: CSIRO national Fish Collection. License: CC By Attribution. habitats. Little information is known on the status of Spinner Sharks throughout its distribution. Within Australia, fishing pressure is currently well managed. The Spinner Shark is assessed globally as Near Threatened (IUCN) and in Australia as Least Concern (IUCN), while Australian stocks are classified as Sustainable (SAFS). Distribution Within Australia, the Spinner Shark is found across northern Australia, from Walpole (Western Australia), throughout the Northern Territory, Queensland and to southern New South Wales (Last and Stevens 2009). It is distributed throughout the world, including the east coast of the United States, Brazil, Mediterranean Sea, west coast of Central Africa, South Africa, Madagascar and throughout the Indo-Pacific. A recent genetic study detected evidence suggesting there may be multiple, genetically- distinct stocks throughout its Australian range (Geraghty et al. 2013). Stock structure and status There is currently very little information on population size and stock status for the Spinner Shark in Australian waters. A suite of management measures introduced from 2009 have led to a substantial reduction in fishing effort targeting adults in New South Wales waters. -

Marine Reptiles Arne R
Virginia Commonwealth University VCU Scholars Compass Study of Biological Complexity Publications Center for the Study of Biological Complexity 2011 Marine Reptiles Arne R. Rasmessen The Royal Danish Academy of Fine Arts John D. Murphy Field Museum of Natural History Medy Ompi Sam Ratulangi University J. Whitfield iG bbons University of Georgia Peter Uetz Virginia Commonwealth University, [email protected] Follow this and additional works at: http://scholarscompass.vcu.edu/csbc_pubs Part of the Life Sciences Commons Copyright: © 2011 Rasmussen et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Downloaded from http://scholarscompass.vcu.edu/csbc_pubs/20 This Article is brought to you for free and open access by the Center for the Study of Biological Complexity at VCU Scholars Compass. It has been accepted for inclusion in Study of Biological Complexity Publications by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. Review Marine Reptiles Arne Redsted Rasmussen1, John C. Murphy2, Medy Ompi3, J. Whitfield Gibbons4, Peter Uetz5* 1 School of Conservation, The Royal Danish Academy of Fine Arts, Copenhagen, Denmark, 2 Division of Amphibians and Reptiles, Field Museum of Natural History, Chicago, Illinois, United States of America, 3 Marine Biology Laboratory, Faculty of Fisheries and Marine Sciences, Sam Ratulangi University, Manado, North Sulawesi, Indonesia, 4 Savannah River Ecology Lab, University of Georgia, Aiken, South Carolina, United States of America, 5 Center for the Study of Biological Complexity, Virginia Commonwealth University, Richmond, Virginia, United States of America Of the more than 12,000 species and subspecies of extant Caribbean, although some species occasionally travel as far north reptiles, about 100 have re-entered the ocean. -
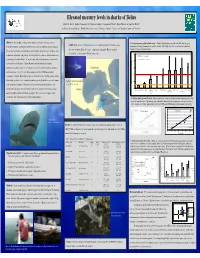
Mercury in Sharks of Belize, 2008
Elevated mercury levels in sharks of Belize David C. Evers 1, Rachel T. Graham 2, Neil Hammerschlag 3, Christopher Perkins 4, Robert Michener 5, and Tim Divoll 1 BioDiversity Research Institute 1, Wildlife Conservation Society 2, University of Miami 3, University of Connecticut 4, and Boston University 5. Abstract: Mercury (Hg) loading in global aquatic ecosystems is a growing concern. 1. Mercury exposure profile by shark species - Highest Hg levels were recorded in the bull, blacktip, great Study Area : A total of 14 distinct locations were sampled in the Gulf of Honduras along Compelling evidence of widespread adverse effects in fish and wildlife populations indicates hammerhead, scalloped hammerhead, and nurse sharks. Lowest Hg levels were recorded in the bonnethead, the coast of southern Belize (red circle). Comparison shark muscle Hg concentrations sharpnose, lemon and sandbar sharks. the rate of transformation to methylmercury is problematic. Long-lived, apex predators such from the U.S. are from southern Florida (white circle). 2.5 as sharks are at high risk to Hg toxicity. We investigated the occurrence of Hg in sharks from USEPA Advisory = 0.3 ug/g, ww coastal waters of southern Belize. In our pilot study, 101 sharks representing 9 species were FDA & WHO Advisory = 1.0 ug/g, ww 2.0 analyzed for muscle Hg levels. Highest Hg levels were recorded in bull, blacktip, hammerhead, and nurse sharks. Lowest Hg levels were recorded in bonnethead, sharpnose, 1.5 and lemon sharks. Over 88% of the sharks sampled exceeded USEPA human health 1.0 consumption standards. Muscle Hg strongly correlated with size for blacktip and nurse sharks. -

Marine Protected Species Identification Guide
Department of Primary Industries and Regional Development Marine protected species identification guide June 2021 Fisheries Occasional Publication No. 129, June 2021. Prepared by K. Travaille and M. Hourston Cover: Hawksbill turtle (Eretmochelys imbricata). Photo: Matthew Pember. Illustrations © R.Swainston/www.anima.net.au Bird images donated by Important disclaimer The Chief Executive Officer of the Department of Primary Industries and Regional Development and the State of Western Australia accept no liability whatsoever by reason of negligence or otherwise arising from the use or release of this information or any part of it. Department of Primary Industries and Regional Development Gordon Stephenson House 140 William Street PERTH WA 6000 Telephone: (08) 6551 4444 Website: dpird.wa.gov.au ABN: 18 951 343 745 ISSN: 1447 - 2058 (Print) ISBN: 978-1-877098-22-2 (Print) ISSN: 2206 - 0928 (Online) ISBN: 978-1-877098-23-9 (Online) Copyright © State of Western Australia (Department of Primary Industries and Regional Development), 2021. ii Marine protected species ID guide Contents About this guide �������������������������������������������������������������������������������������������1 Protected species legislation and international agreements 3 Reporting interactions ���������������������������������������������������������������������������������4 Marine mammals �����������������������������������������������������������������������������������������5 Relative size of cetaceans �������������������������������������������������������������������������5 -

Sea Snakes by Guy Belleranti
Name: ______________________________ Sea Snakes by Guy Belleranti Did you know that some snakes live in the ocean? These snakes are called sea snakes. There are approximately 60 species of sea snakes. Their muscular bodies have flattened, paddle-like tails that help them swim in the warm coastal waters of the Indian and Pacific oceans. Like all snakes, sea snakes have forked tongues and scales. Also, like snakes that live on land, they shed their skin and breathe air. The way they breathe is different from snakes that live on land, however. Sea snakes breathe through lungs and their skin! This allows them to stay underwater for a long time. How long? An hour, two hours, or sometimes even more, depending on the species. Their nostrils are at the top of their snouts. This helps them to breathe more easily when they're at the surface. Sea snakes also have valves in their nostrils that shut water out when they swim. While all sea snakes are very venomous, most sea snakes have short fangs and mild temperaments unless provoked. Even then, many only give a “dry” bite and do not inject venom. However, the two species of beaked sea snakes are aggressive. Fishermen have been bitten, and even killed, by beaked sea snakes tangled in their nets. Sea snakes are carnivores. They hunt along sandy bottoms, under rocks, in coral reefs, and in brackish mangrove swamps for fish, fish eggs, mollusks, and crustaceans. Most sea snakes give birth to live young who then swim away. The majority of sea snakes stay in the sea and are very clumsy on land. -

An Overview of Shark Utilisation in the Coral Triangle Region (PDF, 550
WORKING TOGETHER FOR SUSTAINABLE SHARK FISHERIES AN OVERVIEW OF SHARK UTILISATION IN THE CORAL TRIANGLE REGION Written by Mary Lack Director, Shellack Pty Ltd Glenn Sant Fisheries Programme Leader, TRAFFIC & Senior Fellow, ANCORS Published in September 2012 This report can be downloaded from wwf.panda.org/coraltriangle Citation Lack M. and Sant G. (2012). An overview of shark utilisation in the Coral Triangle region. TRAFFIC &WWF. Photo cover © naturepl.com / Jeff Rotman / WWF-Canon Thanks to the Rufford Lang Foundation for supporting the development of this publication 2 An Overview Of Shark Utilisation In The Coral Triangle Region ACRONYMS ASEAN Association of Southeast Asian Nations BFAR Bureau of Fisheries and Aquatic Resources (the Philippines) CCSBT Commission for the Conservation of Southern Bluefin Tuna CITES Convention on International Trade in Endangered Species of Wild Fauna and Flora CMM Conservation and Management Measure CMS Convention on Migratory Species of Wild Animals CNP Co-operating Non-Contracting party COFI Committee on Fisheries (of FAO) CoP Conference of the Parties (to CITES) EEZ Exclusive Economic Zone EU European Union FAO Food and Agriculture Organization of the United Nations IOTC Indian Ocean Tuna Commission IPOA-Sharks International Plan of Action for the Conservation and Management of Sharks IUU Illegal, Unreported and Unregulated (fishing) MoU Memorandum of Understanding on the Conservation of Migratory Sharks (CMS) nei Not elsewhere included NPOA-Sharks National Plan of Action for the Conservation and