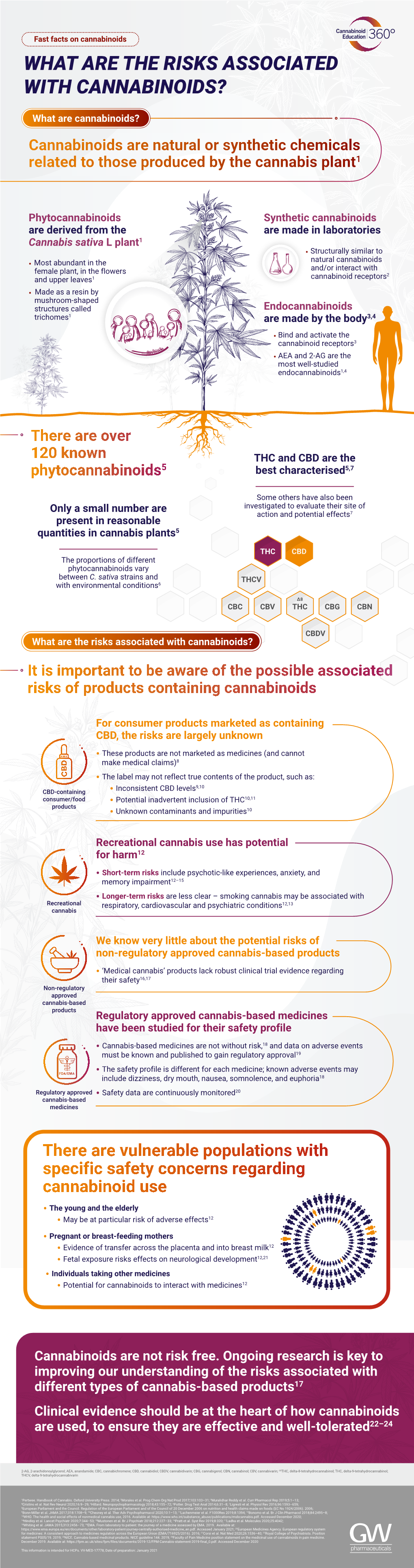
What Are the Risks of Cannabinoids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Targeting the Endocannabinoid System to Reduce Nociception
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2011 Targeting the endocannabinoid system to reduce nociception Lamont Booker Virginia Commonwealth University Follow this and additional works at: https://scholarscompass.vcu.edu/etd Part of the Medical Pharmacology Commons © The Author Downloaded from https://scholarscompass.vcu.edu/etd/2419 This Dissertation is brought to you for free and open access by the Graduate School at VCU Scholars Compass. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. Targeting the Endocannabinoid System to Reduce Nociception A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy at Virginia Commonwealth University. By Lamont Booker Bachelor’s of Science, Fayetteville State University 2003 Master’s of Toxicology, North Carolina State University 2005 Director: Dr. Aron H. Lichtman, Professor, Pharmacology & Toxicology Virginia Commonwealth University Richmond, Virginia April 2011 Acknowledgements The author wishes to thank several people. I like to thank my advisor Dr. Aron Lichtman for taking a chance and allowing me to work under his guidance. He has been a great influence not only with project and research direction, but as an excellent example of what a mentor should be (always willing to listen, understanding the needs of each student/technician, and willing to provide a hand when available). Additionally, I like to thank all of my committee members (Drs. Galya Abdrakmanova, Francine Cabral, Sandra Welch, Mike Grotewiel) for your patience and willingness to participate as a member. Our term together has truly been memorable! I owe a special thanks to Sheryol Cox, and Dr. -

Cannabinoid As Potential Aromatase Inhibitor Through Molecular Modeling and Screening for Anti-Cancer Activity
Cannabinoid as Potential Aromatase Inhibitor Through Molecular Modeling and Screening for Anti-Cancer Activity Sudipta Baroi1, Achintya Saha2, Ritesh Bachar3 and Sitesh C Bachar4 1Department of Pharmaceutical Technology, Faculty of Pharmacy, University of Dhaka Dhaka-1000, Bangladesh 2Department of Chemical Technology, Pharmaceutical & Fine Chemical Technology Division University of Calcutta, India 3Department of Pharmacy, School of Science and Engineering, University of Information Technology and Sciences (UITS), Dhaka-1212, Bangladesh 4Department of Pharmacy, Faculty of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh (Received: April 15, 2020; Accepted: June 2, 2020; Published (web): June 28, 2020) ABSTRACT: Inhibition of aromatase (CYTP450), a key enzyme in the estrogen biosynthesis, could result in regression of estrogen-dependent tumors and even prevent the promotion of breast cancer. The present research has been designed for searching a potent chemical moiety from natural sources to inhibit aromatase enzyme, the over- functionality of which causes the breast cancer. Cannabis sativa contains a very much promising group of cannabinoids with more than 66 compounds with reported anticancer property and for the search of a target specific potent aromatase inhibitor, 61 cannabinoids from C. sativa were selected. The Structures Data File (SDF) of these ligand molecules were subjected to docking studies at the binding site of aromatase X-ray crystallographic structure based on lower resolution of the protein crystal structure and higher docking accuracy, predicted by calculating the correlation between experimental activities and Glide dock scores and compared with the standard aromatase ligand androstenedione and aromatase inhibitor fadrozole with existing drug for breast cancer treatment. The best docked pose of each ligand was selected on the basis of the highest dock score related to the binding free energies of the internal dataset compounds as compared to their observed activities. -

What Is Delta-8 THC?? Cannabinoid Chemistry 101
What is Delta-8 THC?? Cannabinoid Chemistry 101 National Conference on Weights and Measures Annual Meeting - Rochester, NY Matthew D. Curran, Ph.D. July 21, 2021 Disclaimer Just to be clear… • I am a chemist and not a lawyer so: • This presentation will not discuss the legal aspects of Δ8-THC or DEA’s current position. • This presentation will not discuss whether Δ8-THC is considered “synthetic” or “naturally occurring.” • This is not a position statement on any issues before the NCWM. • Lastly, this should only be considered a scientific sharing exercise. Florida Department of Agriculture and Consumer Services 2 Cannabis in Florida Cannabis Syllabus • What is Cannabis? • “Mother” Cannabinoid • Decarboxylation • Relationship between CBD and THC • What does “Total” mean? • Dry Weight vs. Wet Weight • What does “Delta-9” mean? • Relationship between “Delta-8” and “Delta-9” • CBD to Delta-8 THC • Cannabinoid Chemistry 202… Florida Department of Agriculture and Consumer Services 3 Cannabis Cannabis • Cannabis sativa is the taxonomic name for the plant. • The concentration of Total Δ9-Tetrahydrocannabinol (Total Δ9-THC) is critical when considering the varieties of Cannabis sativa. • Hemp – (Total Δ9-THC) 0.3% or less • Not really a controversial term, “hemp” • Marijuana/cannabis – (Total Δ9-THC) Greater than 0.3% • Controversial term, “marijuana” • Some states prohibit the use of this term whereas some states have it in their laws. • Some states use the term “cannabis.” • Not italicized • Lower case “c” Florida Department of Agriculture and -

Roles of Cannabidiol in Reversing Proteinopathies
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 21 May 2020 doi:10.20944/preprints202005.0340.v1 Review Roles of Cannabidiol in Reversing Proteinopathies Raju Dash1, Md. Chayan Ali2†, Israt Jahan3†, Yeasmin Akter Munni1†, Sarmistha Mitra4, Md. Abdul Hannan1,5, Binod Timalsina1, Diyah Fatimah Oktaviani1, Ho Jin Choi1, Il Soo Moon1* 1Department of Anatomy, Dongguk University College of Medicine, Gyeongju 38066, Republic of Korea 2Department of Biotechnology and Genetic Engineering, Faculty of Biological Sciences, Islamic University, Kushtia 7003, Bangladesh 3Department of Pharmacy, Faculty of Life and Earth Sciences, Jagannath University, Dhaka 1100, Bangladesh. 4Plasma Bioscience Research Center and Department of Plasma Bio display, Kwangwoon University, Seoul 01897, Republic of Korea 5Department of Biochemistry and Molecular Biology, Bangladesh Agricultural University, Mymensingh 2202, Bangladesh †These authors have contributed equally. *Corresponding author: Il Soo Moon Mailing address: Department of Anatomy, Dongguk University College of Medicine, 123 Dongdae-ro, Gyeongju 38066, Republic of Korea Phone: +82-54-770-2414 Fax: +82-54-770-2447 Email: [email protected] Abstract: Cannabidiol is a well-known non-psychotropic phytocannabinoid from Cannabis sativa, which exerts a broad range of neuropharmacological activities in the central nervous systems. Over the past years, compelling evidence from preclinical and clinical studies support therapeutic potentials of cannabidiol in various neurological disorders, including neurodegenerative diseases. Neurodegenerative diseases are characterized by the accumulation of misfolded or aggregated protein due to the defective protein homeostasis or proteostasis network, termed as proteinopathies. Because of its role in the protein homeostasis network, cannabidiol could be a potent molecule to revert not only age-associated neurodegeneration but also other protein misfolding disorders. -

In Silico Assessment of Drug-Like Properties of Phytocannabinoids in Cannabis Sativa
EDUCATUM JSMT Vol. 4 No. 2 (2017) ISSN 2289-7070 / eISSN 2462-2451 (1-7) https://ejournal.upsi.edu.my/journal/EDSC In Silico Assessment of Drug-Like Properties of Phytocannabinoids in Cannabis Sativa Shakinaz Desa1*, Asiah Osman2, and Richard Hyslop3 1Department of Biology, Universiti Pendidikan Sultan Idris, Malaysia, 2Natural Product Division, Forest Research Institute Malaysia, 3Department of Chemistry and Biochemistry, University of Northern Colorado, USA *Corresponding author: [email protected] Abstract This study investigated drug-like properties of phytocannabinoids in Cannabis sativa using an in silico study. We report sixteen phytocannabinoids: cannabidiol (CBD), cannabidiolic acid (CBDA), cannabinol (CBN), cannabichromene (CBC), cannabigerol (CBG), cannabicyclol (CBL), cannabivarin (CBV), cannabidivarin (CBDV), cannabichromevarin (CBCV), cannabigerovarin (CBGV), cannabinodiol (CBDL), cannabielsoin (CBE), cannabitriol (CBT), Δ9-tetrahydrocannabinol (Δ9-THC), Δ9-tetrahydrocannabivarin (Δ9-THCV), and Δ8-tetrahydrocannabinol (Δ8-THC). All chemical structures and properties were obtained from PubChem Compound, National Center for Biotechnology Information, U.S. National Library of Medicine. Molinspiration was used for the calculation of molecular properties and bioactivity score. The parameters were molecular weight (MW), number of hydrogen acceptor (HBA), number of hydrogen donor (HBD), partition coefficient (cLogP), polar surface area (PSA) and number of rotatable bonds (NROTB). We predicted bioactivity scores for G Protein-Coupled Receptors (GPCR) ligand, ion channel modulator, kinase inhibitor, nuclear receptor ligand, protease inhibitor and enzyme inhibitor. Lipinski’s rule was used as reference to determine the drug-like properties of the phytocannabinoids. All compounds have MW<500, HBA<10, HBD<5, TPSA<140Å2 and NRTOB<10. Bioactivity score showed an active or moderately active in all compounds. -

Altered Motor Development Following Late Gestational Alcohol and Cannabinoid
bioRxiv preprint doi: https://doi.org/10.1101/513713; this version posted January 7, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Altered Motor Development Following Late Gestational Alcohol and Cannabinoid Exposure in Rats Kristen R. Breit, Brandonn Zamudio, & Jennifer D. Thomas Department of Psychology, Center for Behavioral Teratology, San Diego State University, San Diego, CA, 92120, USA Please address correspondence to: Kristen R. Breit, Ph.D. Email: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/513713; this version posted January 7, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 2 Abstract Cannabis is the most commonly used illicit drug among pregnant women, and rates are likely to increase given recent legalization. In addition, half of pregnant women who report consuming cannabis also report drinking alcohol. However, little is known about the consequences of prenatal cannabis alone or combination with alcohol, particularly with cannabis products that are continually increasing in potency of the primary psychoactive constituent in cannabis, Δ9-tetrahydrocannabinol (THC). The current study investigated the effects of early exposure to cannabinoids during the brain growth spurt on early physical and motor development alone (Experiment 1) or in combination with alcohol (Experiment 2). In Experiment 1, Sprague-Dawley rat pups were exposed to a cannabinoid receptor agonist (CP-55,940 [CP]; 0.1, 0.25, 0.4 mg/kg/day), the drug vehicle, or a saline control from postnatal days (PD) 4-9. -

Fast Detection of 10 Cannabinoids by RP-HPLC-UV Method in Cannabis Sativa L
molecules Article Fast Detection of 10 Cannabinoids by RP-HPLC-UV Method in Cannabis sativa L. Mara Mandrioli 1, Matilde Tura 1, Stefano Scotti 2 and Tullia Gallina Toschi 1,* 1 Department of Agricultural and Food Sciences, Alma Mater Studiorum-University of Bologna, Viale Fanin 40, 40127 Bologna, Italy; [email protected] (M.M.); [email protected] (M.T.) 2 Shimadzu Italia, Via G. B. Cassinis 7, 20139 Milano, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-051-209-6010 Academic Editor: Marcello Locatelli Received: 10 May 2019; Accepted: 31 May 2019; Published: 4 June 2019 Abstract: Cannabis has regained much attention as a result of updated legislation authorizing many different uses and can be classified on the basis of the content of tetrahydrocannabinol (THC), a psychotropic substance for which there are legal limitations in many countries. For this purpose, accurate qualitative and quantitative determination is essential. The relationship between THC and cannabidiol (CBD) is also significant as the latter substance is endowed with many specific and non-psychoactive proprieties. For these reasons, it becomes increasingly important and urgent to utilize fast, easy, validated, and harmonized procedures for determination of cannabinoids. The procedure described herein allows rapid determination of 10 cannabinoids from the inflorescences of Cannabis sativa L. by extraction with organic solvents. Separation and subsequent detection are by RP-HPLC-UV. Quantification is performed by an external standard method through the construction of calibration curves using pure standard chromatographic reference compounds. The main cannabinoids dosed (g/100 g) in actual samples were cannabidiolic acid (CBDA), CBD, and D9-THC (Sample L11 CBDA 0.88 0.04, CBD 0.48 0.02, D9-THC 0.06 0.00; Sample L5 CBDA ± ± ± 0.93 0.06, CBD 0.45 0.03, D9-THC 0.06 0.00). -

Table of Natural Cannabinoids
Table of Natural Cannabinoids Scientific research continues to develop and further identify individual cannabinoids in cannabis strains and how they affect symptoms of illnesses suffered by patients. The table below identifies the chemical properties of the natural cannabinoids found in the average strains of cannabis. Levels of each of these chemicals will vary with varietal strain, growing method, and plant age. Individual cannabinoids can be enhanced or eliminated depending on need. Cannabigerol- type (CBG) Cannabigerol Cannabigerol Cannabigerovarin (E)-CBG-C monomethyl (E)-CBGV-C 5 Cannabinerolic 3 ether acid A (E)-CBGM-C 5 (Z)-CBGA-C A A 5 Cannabigerolic Cannabigerolic Cannabigerovarinic acid acid A acid A A (E)-CBGA-C5 A monomethyl (E)-CBGVA-C3 A ether (E)-CBGAM- C5 A Cannabichrom ene-type (CBC) (±)- (±)- Cannabichromen (±)- Cannabivarichromene, (±)- e Cannabichrome (±)- Cannabichrome CBC-C5 nic acid A Cannabichromevarin varinic CBCA-C5 A CBCV-C3 acid A CBCVA-C3 A Cannabidiol- type (CBD) 1 | Page (−)-Cannabidiol Cannabidiol Cannabidiol-C4 (−)- Cannabidiorc CBD-C5 momomethyl CBD-C4 Cannabidivarin ol ether CBDV-C3 CBD-C1 CBDM-C5 Cannabidiolic Cannabidivarini acid c acid CBDA-C5 CBDVA-C3 Cannabinodiol- type (CBND) Cannabinodiol Cannabinodivar CBND-C5 in CBND-C3 Tetrahydrocan nabinol-type (THC) 9 9 9 Δ - Δ - Δ - Δ9- Tetrahydrocanna Tetrahydrocan Tetrahydrocannabivarin 9 Tetrahydrocan binol nabinol-C4 Δ -THCV-C3 9 9 nabiorcol Δ -THC-C5 Δ -THC-C4 9 Δ -THCO-C1 9 9 Δ -Tetrahydro- Δ9-Tetrahydro- Δ -Tetrahydro- Δ9-Tetrahydro- cannabinolic -

Beta-Caryophyllene Is a Dietary Cannabinoid
Beta-caryophyllene is a dietary cannabinoid Ju¨ rg Gertsch*†, Marco Leonti‡§, Stefan Raduner*§, Ildiko Racz¶, Jian-Zhong Chenʈ, Xiang-Qun Xieʈ, Karl-Heinz Altmann*, Meliha Karsak¶, and Andreas Zimmer¶ *Institute of Pharmaceutical Sciences, Department of Chemistry and Applied Biosciences, Eidgeno¨ssische Technische Hochschule (ETH) Zurich, 8092 Zu¨rich, Switzerland; ‡Dipartimento Farmaco Chimico Tecnologico, University of Cagliari, 01924 Cagliari, Italy; ¶Department of Molecular Psychiatry, University of Bonn, 53115 Bonn Germany; and ʈDepartment of Pharmaceutical Sciences, University of Pittsburgh, Pittsburgh, PA 15260 Edited by L. L. Iversen, University of Oxford, Oxford, United Kingdom, and approved May 6, 2008 (received for review April 14, 2008) The psychoactive cannabinoids from Cannabis sativa L. and the O 5 arachidonic acid-derived endocannabinoids are nonselective nat- 6 12 ural ligands for cannabinoid receptor type 1 (CB1) and CB2 recep- 3 tors. Although the CB1 receptor is responsible for the psychomodu- 7 4 latory effects, activation of the CB2 receptor is a potential 8 2 therapeutic strategy for the treatment of inflammation, pain, 9 1 13 H H H H H H atherosclerosis, and osteoporosis. Here, we report that the wide- 11 10 spread plant volatile (E)--caryophyllene [(E)-BCP] selectively binds 15 nM) and that it is a functional CB2 4 ؎ 155 ؍ to the CB2 receptor (Ki 14 agonist. Intriguingly, (E)-BCP is a common constituent of the (E)-BCP (Z)-BCP BCP oxide α-humulene essential oils of numerous spice and food plants and a major component in Cannabis. Molecular docking simulations have iden- Fig. 1. Caryophyllane- and humulane-type sesquiterpenes found in C. sativa tified a putative binding site of (E)-BCP in the CB receptor, and numerous other plants. -

Cardiovascular Effects of Marijuana and Synthetic Cannabinoids: the Good, the Bad, and the Ugly
REVIEWS PREVENTION OF CVD Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad, and the ugly Pal Pacher1, Sabine Steffens2, György Haskó3, Thomas H. Schindler4 and George Kunos5 Abstract | Dysregulation of the endogenous lipid mediators endocannabinoids and their G‑protein‑coupled cannabinoid receptors 1 and 2 (CB1R and CB2R) has been implicated in a variety of cardiovascular pathologies. Activation of CB1R facilitates the development of cardiometabolic disease, whereas activation of CB2R (expressed primarily in immune cells) exerts anti-inflammatory effects. The psychoactive constituent of marijuana, Δ9-tetrahydrocannabinol (THC), is an agonist of both CB1R and CB2R, and exerts its psychoactive and adverse cardiovascular effects through the activation of CB1R in the central nervous and cardiovascular systems. The past decade has seen a nearly tenfold increase in the THC content of marijuana as well as the increased availability of highly potent synthetic cannabinoids for recreational use. These changes have been accompanied by the emergence of serious adverse cardiovascular events, including myocardial infarction, cardiomyopathy, arrhythmias, stroke, and cardiac arrest. In this Review, we summarize the role of the endocannabinoid system in cardiovascular disease, and critically discuss the cardiovascular consequences of marijuana and synthetic cannabinoid use. With the legalization of marijuana for medicinal purposes and/or recreational use in many countries, physicians should be alert to the possibility -

Phytocannabinoids
Phytocannabinoids More than 100 structurally and physiologically distinct cannabinoid compounds are unique to plants of the genus Cannabis, known collectively as phytocannabinoids. Cayman Chemical offers authentic reference standards for the most prominent of these phytocannabinoids, as well as their metabolites, from our ISO/IEC 17025 and ISO 17034 certified labs. We are working diligently to introduce many more notable phytocannabinoids. If you cannot find a particular compound of interest in our catalog, please contact us for a custom synthesis estimate. Primary Active Constituents Item No. Product Name Metabolites ISO60156 Cannabidiol (CRM)* Item No. Product Name ISO60158 Δ8-THC (CRM)* 21667 (±)-11-hydroxy-Δ9-THC (CRM)* ISO60157 Δ9-THC (CRM)* 20754 (±)-11-nor-9-carboxy-Δ9-THC (CRM)* *Isotopically labeled standard available *Isotopically labeled standard available Naturally Occurring Acids THC Isomers Item No. Product Name Item No. Product Name 9001573 (±)-Cannabichromenic Acid (dicyclohexylamine salt) 25707 (6aR,9S)-Δ10-THC 18090 Cannabidiolic Acid (CRM) 26528 9(R)-Δ6a,10a-THC 20019 Cannabigerolic Acid (CRM) 26529 9(S)-Δ6a,10a-THC ISO60175 THCA-A (CRM) 26283 Varinolic Acid Discover Our Multi-Component Varinol Series Phytocannabinoid CRM Mixtures Item No. Product Name Available to quantify up to 10 21974 (±)-Cannabichromevarin (CRM) prevalent phytocannabinoids 20165 Cannabidivarin (CRM) Learn more 21664 Cannabivarin on Page 2 18091 Tetrahydrocannabivarin (CRM) of this Other Cannabinoids of Interest brochure Item No. Product Name ISO60163 -

Regulate Cannabinoid Products. (Public)
GENERAL ASSEMBLY OF NORTH CAROLINA SESSION 2021 H 1 HOUSE BILL 818 Short Title: Regulate Cannabinoid Products. (Public) Sponsors: Representatives Sasser, Humphrey, and McNeely (Primary Sponsors). For a complete list of sponsors, refer to the North Carolina General Assembly web site. Referred to: Agriculture, if favorable, Rules, Calendar, and Operations of the House May 5, 2021 1 A BILL TO BE ENTITLED 2 AN ACT TO DIRECT THE DEPARTMENT OF AGRICULTURE AND CONSUMER 3 SERVICES TO ESTABLISH A VOLUNTARY LICENSING PROGRAM FOR 4 CANNABINOID-RELATED COMPOUNDS. 5 The General Assembly of North Carolina enacts: 6 SECTION 1. G.S. 106-121 reads as rewritten: 7 "§ 106-121. Definitions and general consideration. 8 For the purpose of this Article: 9 (1) The term "advertisement" means all representations disseminated in any 10 manner or by any means, other than by labeling, for the purposes of inducing, 11 or which are likely to induce, directly or indirectly, the purchase of food, 12 drugs, devices or cosmetics. 13 (1a) The term "cannabinoid-related compounds" means any phytocannabinoid 14 found in hemp, including, but not limited to, tetrahydrocannabinol (THC), 15 tetrahydrocannabinolic acid (THCA), cannabidiol (CBD), cannabidiolic acid 16 (CBDA), cannabinol (CBN), cannabigerol (CBG), cannabichromene (CBC), 17 cannabicyclol (CBL), cannabivarin (CBV), tetrahydrocannabivarin (THCV), 18 cannabidivarin (CBDV), cannabichromevarin (CBCV), cannabigerovarin 19 (CBGV), cannabigerol monomethyl ether (CBGM), cannabielsoin (CBE), or 20 cannabicitran (CBT). Cannabinoids do not include synthetic cannabinoids. 21 (1a)(1b) The term "color" includes black, white, and intermediate grays. 22 (1b)(1c) The term "color additive" means a material which: 23 …." 24 SECTION 2. G.S.