Fischer Projection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Structures of Monosaccharides Hemiacetals
Structures of Monosaccharides Hemiacetals • Although, the open chain structures of monosaccharides are consistent with the chemistry of carbohydrates, in reality they are oversimplifications of the true structure of carbohydrates. • It is common knowledge that aldehydes react with alcohols to form hemiacetals. In cases where a molecule is a hydroxyaldehyde such as 4- hydroxybutanal or 5-hydroxypentanal, cyclic hemiacetals result. 9:47 AM 1 Structures of Monosaccharides Hemiacetals • Aldoses often contain an aldehyde group and several hydroxyl groups as part of the same molecule; they have a greater tendency of forming cyclic hemiacetals. In fact, in aqueous solution carbohydrates exist almost exclusively in the ring-closed form At equilibrium, the linear aldehyde or ketone structure represents less than 1% of the sugar present. • Five and six-membered rings are thermodynamically more stable than their corresponding four and seven membered rings, since they are less strained. • Five- (furanoses) and six-membered cyclic hemiacetals (pyranoses) are often more stable than their open-chain forms. In particular the six-membered rings which can adopt a chair conformation are 9:47 AM 2 essentially free from all types of strains. Structures of Monosaccharides Evidence for Existence of Monosacharides as Hemiacetals What physical, chemical and spectroscopic evidence support the existence of monosaccharide sugars as cyclic hemi-acetals. (a) Two anomers of glucose capable of existing independently with different physical (melting points and specific optical rotation) and chemical properties can be obtained by recrystallization. (b) the 1H-NMR and IR-spectra of solutions of pure sugars show the presence of mixtures (anomeric hemiacetals) and absence of an aldehydic peak is a sufficient indicator that the sugars exist in some other form other than the open-chain form. -

Uvic Thesis Template
Insight into the Functionality of an Unusual Glycoside Hydrolase from Family 50 by Kaleigh Giles BSc, Brock University, 2011 A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE in the Department of Biochemistry and Microbiology Kaleigh Giles, 2014 University of Victoria All rights reserved. This thesis may not be reproduced in whole or in part, by photocopy or other means, without the permission of the author. ii Supervisory Committee Insight into the Functionality of an Unusual Glycoside Hydrolase from Family 50 by Kaleigh Giles BSc, Brock University, 2011 Supervisory Committee Dr. Alisdair B. Boraston, Department of Biochemistry and Microbiology Supervisor Dr. Martin J. Boulanger (Department of Biochemistry and Microbiology) Departmental Member Dr. Fraser Hof (Department of Chemistry) Outside Member iii Abstract Supervisory Committee Dr. Alisdair B. Boraston, Department of Biochemistry and Microbiology Supervisor Dr. Martin J. Boulanger, Department of Biochemistry and Microbiology Departmental Member Dr. Fraser Hof, Department of Chemistry O utside Member Agarose and porphyran are related galactans that are only found within red marine algae. As such, marine microorganisms have adapted to using these polysaccharides as carbon sources through the acquisition of unique Carbohydrate Active enZymes (CAZymes). A recent metagenome study of the microbiomes from a Japanese human population identified putative CAZymes in several bacterial species, including Bacteroides plebeius that have significant amino acid sequence similarity with those from marine bacteria. Analysis of one potential CAZyme from B. plebeius (BpGH50) is described here. While displaying up to 30% sequence identity with β-agarases, BpGH50 has no detectable agarase activity. Its crystal structure reveals that the topology of the active site is much different than previously characterized agarases, while containing the same core catalytic machinery. -
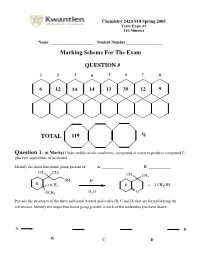
Marking Scheme for the Exam
Chemistry 2420 S10 Spring 2005 Term Exam #1 110 Minutes Name: ______________________ Student Number___________________ Marking Scheme For The Exam QUESTION # 1 2 3 4 5 6 7 8 6 12 14 14 13 39 12 9 TOTAL 119 % Question 1. (6 Marks) Under mildly acidic conditions, compound A reacts to produce compound E plus two equivalents of methanol. Identify the main functional group present in: A: ___________ E: ___________ CH3 CH3 CH 3 CH3 + NH2 H A OCH3 E + 2 CH3OH H O OCH3 2 N Provide the structures of the three additional neutral molecules (B, C and D) that are formed during the conversion. Identify the major functional group present in each of the molecules you have drawn. A E B C D Question 2. (12 Marks) Circle the structure that will best satisfy the given information. - the compound able to undergo an intramolecular cyclization to form a hemiacetal H H HO H HO OH O O O - the compound able to undergo an intramolecular cyclization to form an enamine H H H Me2N MeHN H2N O O O + - the produc t formed from treatment of 2-butanone with NaBD4 followed by a H3O work-up DO H HO D DO D HO H - the compound that would most likely be coloured O H O O - the compound capable of undergoing mutarotation O O O OH OCH3 OH CH3 H CH 3 - the compound that would not react with MeMgBr O O O OH OCH3 OH CH3 H CH3 - the compound bes t described as “anti-aromatic”: - the most stable carbocation + + + Cl Cl Cl OCH 3 H NO2 H H H Question 3. -

Glycoconjugates
Background Information on Glycoconjugates Richard D. Cummings, Ph.D. Director, National Center for Functional Glycomics Professor Department of Surgery Beth Israel Deaconess Medical Center Harvard Medical School Boston, MA 02114 Tel: (617) 735-4643 e-mail: [email protected] For General Reference On-Line See: Essentials of Glycobiology (2nd Edition) Varki, Cummings, Esko, Freeze, Stanley, Bertozzi, Hart and Etzler) http://www.ncbi.nlm.nih.gov/books/NBK1908/ Mammalian Cells are Covered with Glycoconjugates GLYCOSAMINOGLYCANS/ GLYCOPROTEINS PROTEOGLYCANS GLYCOLIPIDS NUCLEAR/CYTOPLASMIC GLYCOPROTEINS 2 Mammalian Glycoconjugates are Recognized by a Wide Variety of Specific Proteins GLYCAN-BINDING PROTEIN (GBP) GBP ANTIBODY TOXIN GBP GBP VIRUS 7 ANTIBODY GBP MICROBE TOXIN 3 Glycosylation Pathways 4 Glycosylation Pathways 5 Glycoconjugates, Which are Molecules Containing Sugars (Monosaccharides) Linked Within Them, are the Major Constituents of Animal Cell Membranes (Glycocalyx) and Secreted Material: See Different Classes of Glycoconjugates Below in Red Boxes PROTEOGLYCANS GLYCOSAMINOGLYCANS GLYCOSAMINOGLYCANS GLYCOPROTEINS GPI-ANCHORED GLYCOPROTEINS GLYCOLIPIDS outside Cell Membrane cytoplasm Essentials of Glycobiology, 3rd Edition CYTOPLASMIC GLYCOPROTEINS Chapter 1, Figure 6 Glycans are as Ubiquitous as DNA/RNA and Appear to Represent Greater Molecular Diversity 7 Big Picture: Nucleotide Sugars Connection of • UDP-Glc, • UDP-Gal, • UDP-GlcNAc, Glycoconjugate • UDPGalNAc, • UDP-GlcA, Biosynthesis • UDP-Xyl, • GDP-Man, • GDP-Fuc, to Intermediary • CMP-Neu5Ac used for synthesizing Metabolism glycoconjugates, e.g, glycoproteins & glycolipids 8 Important Topics to Consider 1. The different types of monosaccharides found in animal cell glycoconjugates 2. The different types of glycoconjugates and their differences, e.g. glycoproteins, glycolipids 3. The nucleotide sugars, glycosyltransferases, glycosidases, transporters, endoplasmic reticulum, and Golgi in terms of their roles in glycoconjugate biosynthesis and turnover 4. -

Collagen-Based Matrix Matrix Auf Der Basis Von Kollagen Matrice À Base De Collagène
Europäisches Patentamt *EP000693523B1* (19) European Patent Office Office européen des brevets (11) EP 0 693 523 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.7: C08H 1/06, A61L 27/00, of the grant of the patent: A61L 31/00 20.11.2002 Bulletin 2002/47 (21) Application number: 95111260.6 (22) Date of filing: 18.07.1995 (54) Collagen-based matrix Matrix auf der Basis von Kollagen Matrice à base de collagène (84) Designated Contracting States: • Noff, Matityahu AT BE CH DE DK ES FR GB GR IE IT LI LU MC NL Rehovot 76228 (IL) PT SE (74) Representative: Grünecker, Kinkeldey, (30) Priority: 19.07.1994 IL 11036794 Stockmair & Schwanhäusser Anwaltssozietät Maximilianstrasse 58 (43) Date of publication of application: 80538 München (DE) 24.01.1996 Bulletin 1996/04 (56) References cited: (73) Proprietor: COL-BAR R & D LTD. DE-A- 4 302 708 FR-A- 2 679 778 Ramat-Gan 52290 (IL) US-A- 4 971 954 (72) Inventors: Remarks: • Pitaru, Sandu The file contains technical information submitted Tel-Aviv 62300 (IL) after the application was filed and not included in this specification Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. Notice of opposition shall be filed in a written reasoned statement. It shall not be deemed to have been filed until the opposition fee has been paid. (Art. 99(1) European Patent Convention). EP 0 693 523 B1 Printed by Jouve, 75001 PARIS (FR) EP 0 693 523 B1 Description [0001] The present invention concerns a collagen-based matrix and devices comprising this matrix. -

REFERENCE ONLY UNIVERSITY of LONDON THESIS This Thesis
REFERENCE ONLY UNIVERSITY OF LONDON THESIS Degree Year Name of Author 0" * COPYRIGHT This is a thesis accepted for a Higher Degree of the University of London. It is an unpublished typescript and the copyright is held by the author. All persons consulting the thesis must read and abide by the Copyright Declaration below. COPYRIGHT DECLARATION I recognise that the copyright of the above-described thesis rests with the author and that no quotation from it or information derived from it may be published without the prior written consent of the author. LOANS Theses may not be lent to individuals, but the Senate House Library may lend a copy to approved libraries within the United Kingdom, for consultation solely on the premises of those libraries. Application should be made to: Inter-Library Loans, Senate House Library, Senate House, Malet Street, London WC1E 7HU. REPRODUCTION University of London theses may not be reproduced without explicit written permission from the Senate House Library. Enquiries should be addressed to the Theses Section of the Library. Regulations concerning reproduction vary according to the date of acceptance of the thesis and are listed below as guidelines. A. Before 1962. Permission granted only upon the prior written consent of the author. (The Senate House Library will provide addresses where possible). B. 1962- 1974. In many cases the author has agreed to permit copying upon completion of a Copyright Declaration. C. 1975 - 1988. Most theses may be copied upon completion of a Copyright Declaration. D. 1989 onwards. Most theses may be copied. This thesis comes within category D. -

Intermediate Transfer Type Ink Jet Recording Method
Europaisches Patentamt J European Patent Office © Publication number: 0 606 490 A1 Office europeen des brevets EUROPEAN PATENT APPLICATION published in accordance with Art. 158(3) EPC © Application number: 93914949.8 int. ci.5: B41J 2/01 @ Date of filing: 02.07.93 © International application number: PCT/JP93/00914 © International publication number: WO 94/01283 (20.01.94 94/03) © Priority: 02.07.92 JP 175384/92 Inventor: HOSONO, Yoshie 02.07.92 JP 175385/92 Seiko Epson Corporation, 02.07.92 JP 175386/92 3-5, Owa 3-chome 02.07.92 JP 175387/92 Suwa-shi, Nagano 392(JP) 16.10.92 JP 278938/92 Inventor: NAKAMURA, Hiroto 05.11.92 JP 296109/92 Seiko Epson Corporation, 05.11.92 JP 296110/92 3-5, Owa 3-chome 05.11.92 JP 296111/92 Suwa-shi, Nagano 392(JP) 06.01.93 JP 665/93 Inventor: KOIKE, Yoshiyuki Seiko Epson Corporation, © Date of publication of application: 3-5, Owa 3-chome 20.07.94 Bulletin 94/29 Suwa-shi, Nagano 392(JP) Inventor: MATSUZAKI, Makoto @ Designated Contracting States: Seiko Epson Corporation, CH DE FR GB IT LI NL SE 3-5, Owa 3-chome Suwa-shi, Nagano 392(JP) © Applicant: SEIKO EPSON CORPORATION Inventor: UEHARA, Fumie 4-1, Nishishinjuku 2-chome Seiko Shinjuku-ku Tokyo 163(JP) Epson Corporation, 3-5, Owa 3-chome © Inventor: FUJINO, Makoto Suwa-shi, Nagano 392(JP) Seiko Epson Corporation, Inventor: ISHIBASHI, Osamu 3-5, Owa 3-chome Seiko Epson Corporation, Suwa-shi, Nagano 392(JP) 3-5, Owa 3-chome Inventor: KUMAGAI, Toshio Suwa-shi, Nagano 392(JP) Seiko Epson Corporation, 3-5, Owa 3-chome Suwa-shi, Nagano 392(JP) © Representative: Lewin, John Harvey Inventor: TSUKAHARA, Michinari Elkington and Fife Seiko Epson Corporation, Prospect House CO 8 Pembroke Road o 3-5, Owa 3-chome CO Suwa-shi, Nagano 392(JP) Sevenoaks, Kent TN13 1XR (GB) &) INTERMEDIATE TRANSFER TYPE INK JET RECORDING METHOD. -

108B Carbohydrate Activity
CHEM 108B UCSC, Binder CARBOHYDRATE ACTIVITY Structural Conventions – complete #1-6 before the first carbohydrates lecture to facilitate understanding in lecture (due in discussion 2/29-3/4). The last two problems and definitions are due in discussion the following week (3/7-3/11). Use chapter 25 of the McMurry text to learn this as you go but some terms may require a quick google search. Define the terms on the next page as you go along. 1. Draw one example of each of the following types of monosaccharides (there may be several correct answers) and indicate the number of possible stereoisomers while keeping the same D/L configuration. a. D-Aldotriose b. L-Ketotetrose c. L-Aldopentose d. D-Ketohexose e. L-Aldohexose 2. Draw Fischer projections of the following: a. The C2 epimer of D-Glucose b. The C3 epimer of D-Glucose c. The C4 epimer of D-Glucose 3. Each monosaccharide from #1-2 can act as a nucleophile or electrophile. Redraw sugars #1d and #1e and indicate the functional groups that could act as nucleophiles and those that can serve as electrophiles. 4. Draw a skeleton Haworth projection and chair conformation of a D- monosaccharide in closed form: a 6-membered oxygen-containing ring without the hydroxyl groups. 5. Draw Haworth projections for the following (consult Fig 25.3 of McMurry; memorize the structure of D-Glucose for the final exam). What is the relationship between a & b; between a & c? a. α-D-Allopyranose b. β-D-Allopyranose c. α-D-Glucopyranose d. -

Part 1 in Our Series of Carbohydrate Lectures. in This Section, You Will Learn About Monosaccharide Structure
Welcome to Part 1 in our series of Carbohydrate lectures. In this section, you will learn about monosaccharide structure. The building blocks of larger carbohydrate polymers. 1 First, let’s review why learning about carbohydrates is important. Carbohydrates are used by biological systems as fuels and energy resources. Carbohydrates typically provide quick energy and are one of the primary energy storage forms in animals. Carbohydrates also provide the precursors to other major macromolecules within the body, including the deoxyribose and ribose required for nucleic acid biosynthesis. Carbohydrates can also provide structural support and cushioning/shock absorption, as well as cell‐cell communication, identification, and signaling. 2 Carbohydrates, as their name implies, are water hydrates of carbon, and they all have the same basic core formula (CH2O)n and are always found in the ratio of 1 carbon to 2 hydrogens to 1 oxygen (1:2:1) making them easy to identify from their molecular formula. 3 Carbohydrates can be divided into subcategories based on their complexity. The simplest carbohydrates are the monosaccharides which are the simple sugars required for the biosynthesis of all the other carbohydrate types. Disaccharides consist of two monosaccharides that have been joined together by a covalent bond called the glycosidic bond. Oligosaccharides are polymers that consist of a few monosaccharides covalently linked together, and Polysaccharides are large polymers that contain hundreds to thousands of monosaccharide units all joined together by glycosidic bonds. The remainder of this lecture will focus on monosaccharides 4 Monosaccharides all have alcohol functional groups associated with them. In addition they also have one additional functional group, either an aldehyde or a ketone. -

Lipids, Carbohydrates, Nucleobases & DNA
Chemistry 2050 “Introduction to Organic Chemistry” Fall Semester 2011 Dr. Rainer Glaser Examination #5: The Final “Lipids, Carbohydrates, Nucleobases & DNA.” Monday, December 12, 2011, 10 am – 12 pm. Name: Answer Key Question 1. Lipids and Detergents. 20 Question 2. Glyceraldehyde and Carbohydrates. 20 Question 3. Nucleobases of DNA and RNA. 20 Question 4. Ribose and Deoxyribose and Their Phosphate Esters. 20 Question 5. Single and Double Strands of DNA. 20 Total 100 — 1 — Question 1. Lipids and Detergents. (20 points) (a) Draw the line segment drawing of glyceryl distearoleate. This fat is the _TRI-ester formed between one molecule of the triol __GLYCEROL_, two molecules of the fatty acid stearic acid, H3C−(CH2)16−COOH, and one molecule of the unsaturated fatty acid oleic acid, H3C−(CH2)7−CH=CH−(CH2)7−COOH. In your drawing, attach the oleic acid to one of the primary alcohols of the triol. Clearly indicate whether the alkene in oleic acid is cis or trans. To convert this fat into soap, one would cook the fat with aqueous NaOH solution, a process that is called __SAPONIFICATION__. (12 points) (b) A simple micelle is shown schematically on the right. Each circle signifies a _________ (polar, nonpolar) head-group, and each wiggly line signifies a ________ (polar, nonpolar) alkyl chain. In the case of “normal” soap, the head- group is a ___CARBOXYLATE__ anion. Indicate where we would find the associated cations (i.e., sodium cations; draw a few in the scheme). Water is on the __________ (inside, outside) of this micelle and fats will accumulate on the _________ (inside, outside) of this micelle. -

Organic Chemistry by Robert C
(2/94)(6,7,9/95)(8,9/97)(12/99)(1/00) Neuman Chapter 4 Chapter 4 Stereochemistry from Organic Chemistry by Robert C. Neuman, Jr. Professor of Chemistry, emeritus University of California, Riverside [email protected] <http://web.chem.ucsb.edu/~neuman/orgchembyneuman/> Chapter Outline of the Book ************************************************************************************** I. Foundations 1. Organic Molecules and Chemical Bonding 2. Alkanes and Cycloalkanes 3. Haloalkanes, Alcohols, Ethers, and Amines 4. Stereochemistry 5. Organic Spectrometry II. Reactions, Mechanisms, Multiple Bonds 6. Organic Reactions *(Not yet Posted) 7. Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic Substitution 8. Alkenes and Alkynes 9. Formation of Alkenes and Alkynes. Elimination Reactions 10. Alkenes and Alkynes. Addition Reactions 11. Free Radical Addition and Substitution Reactions III. Conjugation, Electronic Effects, Carbonyl Groups 12. Conjugated and Aromatic Molecules 13. Carbonyl Compounds. Ketones, Aldehydes, and Carboxylic Acids 14. Substituent Effects 15. Carbonyl Compounds. Esters, Amides, and Related Molecules IV. Carbonyl and Pericyclic Reactions and Mechanisms 16. Carbonyl Compounds. Addition and Substitution Reactions 17. Oxidation and Reduction Reactions 18. Reactions of Enolate Ions and Enols 19. Cyclization and Pericyclic Reactions *(Not yet Posted) V. Bioorganic Compounds 20. Carbohydrates 21. Lipids 22. Peptides, Proteins, and α−Amino Acids 23. Nucleic Acids ************************************************************************************** -

Structures and Characteristics of Carbohydrates in Diets Fed to Pigs: a Review Diego M
Navarro et al. Journal of Animal Science and Biotechnology (2019) 10:39 https://doi.org/10.1186/s40104-019-0345-6 REVIEW Open Access Structures and characteristics of carbohydrates in diets fed to pigs: a review Diego M. D. L. Navarro1, Jerubella J. Abelilla1 and Hans H. Stein1,2* Abstract The current paper reviews the content and variation of fiber fractions in feed ingredients commonly used in swine diets. Carbohydrates serve as the main source of energy in diets fed to pigs. Carbohydrates may be classified according to their degree of polymerization: monosaccharides, disaccharides, oligosaccharides, and polysaccharides. Digestible carbohydrates include sugars, digestible starch, and glycogen that may be digested by enzymes secreted in the gastrointestinal tract of the pig. Non-digestible carbohydrates, also known as fiber, may be fermented by microbial populations along the gastrointestinal tract to synthesize short-chain fatty acids that may be absorbed and metabolized by the pig. These non-digestible carbohydrates include two disaccharides, oligosaccharides, resistant starch, and non-starch polysaccharides. The concentration and structure of non-digestible carbohydrates in diets fed to pigs depend on the type of feed ingredients that are included in the mixed diet. Cellulose, arabinoxylans, and mixed linked β-(1,3) (1,4)-D-glucans are the main cell wall polysaccharides in cereal grains, but vary in proportion and structure depending on the grain and tissue within the grain. Cell walls of oilseeds, oilseed meals, and pulse crops contain cellulose, pectic polysaccharides, lignin, and xyloglucans. Pulse crops and legumes also contain significant quantities of galacto-oligosaccharides including raffinose, stachyose, and verbascose.