ZELDOX Capsules for Oral Use
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
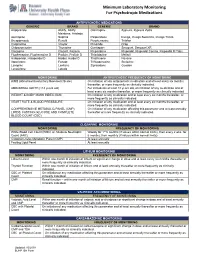
Minimum Laboratory Monitoring for Psychotropic Medications
Minimum Laboratory Monitoring For Psychotropic Medications ANTIPSYCHOTIC MEDICATIONS GENERIC BRAND GENERIC BRAND Aripiprazole Abilify, Abilify Olanzapine Zyprexa, Zyprexa Zydis Maintena, Aristada Asenapine Saphris Paliperidone Invega, Invega Sustenna, Invega Trinza Brexpiprazole Rexulti Perphenazine Trilafon Cariprazine Vraylar Pimozide Orap Chlorpromazine Thorazine Quetiapine Seroquel, Seroquel XR Clozapine Clozaril, Fazaclo Risperidone Risperdal, Risperdal Consta, Risperdal M Tabs Fluphenazine, Fluphenazine D Prolixin, Prolixin D Thioridazine Mellaril Haloperidol, Haloperidol D Haldol, Haldol D Thiothixene Navane Iloperidone Fanapt Trifluoperazine Stelazine Loxapine Loxitane Ziprasidone Geodon Lurasidone Latuda MONITORING ANTIPSYCHOTIC FREQUENCY OF MONITORING AIMS (Abnormal Involuntary Movement Scale) On initiation of any antipsychotic medication and at least every six months thereafter, or more frequently as clinically indicated. ABDOMINAL GIRTH (>18 years old) For individuals at least 18 years old, on initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. WEIGHT & BODY MASS INDEX (BMI) On initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. HEART RATE & BLOOD PRESSSURE On initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. COMPREHENSIVE METABOLIC PANEL (CMP), On initiation of any medication affecting this parameter and at least annually LIPIDS, FASTING -

Schizophrenia Care Guide
August 2015 CCHCS/DHCS Care Guide: Schizophrenia SUMMARY DECISION SUPPORT PATIENT EDUCATION/SELF MANAGEMENT GOALS ALERTS Minimize frequency and severity of psychotic episodes Suicidal ideation or gestures Encourage medication adherence Abnormal movements Manage medication side effects Delusions Monitor as clinically appropriate Neuroleptic Malignant Syndrome Danger to self or others DIAGNOSTIC CRITERIA/EVALUATION (PER DSM V) 1. Rule out delirium or other medical illnesses mimicking schizophrenia (see page 5), medications or drugs of abuse causing psychosis (see page 6), other mental illness causes of psychosis, e.g., Bipolar Mania or Depression, Major Depression, PTSD, borderline personality disorder (see page 4). Ideas in patients (even odd ideas) that we disagree with can be learned and are therefore not necessarily signs of schizophrenia. Schizophrenia is a world-wide phenomenon that can occur in cultures with widely differing ideas. 2. Diagnosis is made based on the following: (Criteria A and B must be met) A. Two of the following symptoms/signs must be present over much of at least one month (unless treated), with a significant impact on social or occupational functioning, over at least a 6-month period of time: Delusions, Hallucinations, Disorganized Speech, Negative symptoms (social withdrawal, poverty of thought, etc.), severely disorganized or catatonic behavior. B. At least one of the symptoms/signs should be Delusions, Hallucinations, or Disorganized Speech. TREATMENT OPTIONS MEDICATIONS Informed consent for psychotropic -

Current P SYCHIATRY
Current p SYCHIATRY N ew Investigators Tips to manage and prevent discontinuation syndromes Informed tapering can protect patients when you stop a medication Sriram Ramaswamy, MD Shruti Malik, MBBS, MHSA Vijay Dewan, MD Instructor, department of psychiatry Foreign medical graduate Assistant professor Creighton University Department of psychiatry Omaha, NE University of Nebraska Medical Center Omaha, NE bruptly stopping common psychotropics New insights on psychotropic A —particularly antidepressants, benzodi- drug safety and side effects azepines, or atypical antipsychotics—can trigger a discontinuation syndrome, with: This paper was among those entered in the 2005 • rebound or relapse of original symptoms Promising New Investigators competition sponsored • uncomfortable new physical and psycho- by the Neuroleptic Malignant Syndrome Information Service (NMSIS). The theme of this year’s competition logical symptoms was “New insights on psychotropic drug safety and • physiologic withdrawal at times. side effects.” To increase health professionals’ awareness of URRENT SYCHIATRY 1 C P is honored to publish this peer- the risk of these adverse effects, this article reviewed, evidence-based article on a clinically describes discontinuation syndromes associated important topic for practicing psychiatrists. with various psychotropics and offers strategies to NMSIS is dedicated to reducing morbidity and anticipate, recognize, and manage them. mortality of NMS by improving medical and psychiatric care of patients with heat-related disorders; providing -

Clinical Review, Adverse Events
Clinical Review, Adverse Events Drug: Carbamazepine NDA: 16-608, Tegretol 20-712, Carbatrol 21-710, Equetro Adverse Event: Stevens-Johnson Syndrome Reviewer: Ronald Farkas, MD, PhD Medical Reviewer, DNP, ODE I 1. Executive Summary 1.1 Background Carbamazepine (CBZ) is an anticonvulsant with FDA-approved indications in epilepsy, bipolar disorder and neuropathic pain. CBZ is associated with Stevens-Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN), closely related serious cutaneous adverse drug reactions that can be permanently disabling or fatal. Other anticonvulsants, including phenytoin, phenobarbital, and lamotrigine are also associated with SJS/TEN, as are members of a variety of other drug classes, including nonsteriodal anti-inflammatory drugs and sulfa drugs. The incidence of CBZ-associated SJS/TEN has been considered “extremely rare,” as noted in current U.S. drug labeling. However, recent publications and postmarketing data suggest that CBZ- associated SJS/TEN occurs at a much higher rate in some Asian populations, about 2.5 cases per 1,000 new exposures, and that most of this increased risk is in individuals carrying a specific human leukocyte antigen (HLA) allele, HLA-B*1502. This HLA-B allele is present in about 5- to 20% of many, but not all, Asian populations, and is also present in about 2- to 4% of South Asians/Indians. The allele is also present at a lower frequency, < 1%, in several other ethnic groups around the world (although likely due to distant Asian ancestry). About 10% of U.S. Asians carry HLA-B*1502. HLA-B*1502 is generally not present in the U.S. -

Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment for Schizophrenia in Adult Patients? Kyle J
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Student Dissertations, Theses and Papers Scholarship 2017 Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients? Kyle J. Knowles Philadelphia College of Osteopathic Medicine Follow this and additional works at: https://digitalcommons.pcom.edu/pa_systematic_reviews Part of the Psychiatry Commons Recommended Citation Knowles, Kyle J., "Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients?" (2017). PCOM Physician Assistant Studies Student Scholarship. 381. https://digitalcommons.pcom.edu/pa_systematic_reviews/381 This Selective Evidence-Based Medicine Review is brought to you for free and open access by the Student Dissertations, Theses and Papers at DigitalCommons@PCOM. It has been accepted for inclusion in PCOM Physician Assistant Studies Student Scholarship by an authorized administrator of DigitalCommons@PCOM. For more information, please contact [email protected]. Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients? Kyle J. Knowles, PA-S A SELECTIVE EVIDENCE BASED MEDICINE REVIEW In Partial Fulfillment of the Requirements For The Degree of Master of Science In Health Sciences- Physician Assistant Department of Physician Assistant Studies Philadelphia College of Osteopathic Medicine Philadelphia, Pennsylvania December 16, 2016 ABSTRACT OBJECTIVE: The objective of this selective EBM review is to determine whether or not “Is Aristada (aripiprazole lauroxil) a safe and effective treatment for schizophrenia in adult patients?” STUDY DESIGN: Review of three randomized controlled studies. All three trials were conducted between 2014 and 2015. DATA SOURCES: One randomized, controlled trial and two randomized, controlled, double- blind trials found via Cochrane Library and PubMed. -

Doxepin Exacerbates Renal Damage, Glucose Intolerance, Nonalcoholic Fatty Liver Disease, and Urinary Chromium Loss in Obese Mice
pharmaceuticals Article Doxepin Exacerbates Renal Damage, Glucose Intolerance, Nonalcoholic Fatty Liver Disease, and Urinary Chromium Loss in Obese Mice Geng-Ruei Chang 1,* , Po-Hsun Hou 2,3, Wei-Cheng Yang 4, Chao-Min Wang 1 , Pei-Shan Fan 1, Huei-Jyuan Liao 1 and To-Pang Chen 5,* 1 Department of Veterinary Medicine, National Chiayi University, 580 Xinmin Road, Chiayi 60054, Taiwan; [email protected] (C.-M.W.); [email protected] (P.-S.F.); [email protected] (H.-J.L.) 2 Department of Psychiatry, Taichung Veterans General Hospital, 1650 Taiwan Boulevard (Section 4), Taichung 40705, Taiwan; [email protected] 3 Faculty of Medicine, National Yang-Ming University, 155 Linong Street (Section 2), Taipei 11221, Taiwan 4 School of Veterinary Medicine, National Taiwan University, 1 Roosevelt Road (Section 4), Taipei 10617, Taiwan; [email protected] 5 Division of Endocrinology and Metabolism, Show Chwan Memorial Hospital, 542 Chung-Shan Road (Section 1), Changhua 50008, Taiwan * Correspondence: [email protected] (G.-R.C.); [email protected] (T.-P.C.); Tel.: +886-5-2732946 (G.-R.C.); +886-4-7256166 (T.-P.C.) Abstract: Doxepin is commonly prescribed for depression and anxiety treatment. Doxepin-related disruptions to metabolism and renal/hepatic adverse effects remain unclear; thus, the underlying mechanism of action warrants further research. Here, we investigated how doxepin affects lipid Citation: Chang, G.-R.; Hou, P.-H.; change, glucose homeostasis, chromium (Cr) distribution, renal impairment, liver damage, and fatty Yang, W.-C.; Wang, C.-M.; Fan, P.-S.; liver scores in C57BL6/J mice subjected to a high-fat diet and 5 mg/kg/day doxepin treatment for Liao, H.-J.; Chen, T.-P. -

Ziprasidone (Geodon®)
Ziprasidone (Geodon®) This sheet talks about exposure to ziprasidone in pregnancy and while breastfeeding. This information should not take the place of medical care and advice from your healthcare provider. What is ziprasidone? Ziprasidone is a medication that has been used to treat bipolar disorder, schizophrenia, and schizoaffective disorder. Ziprasidone belongs to a group of medications called atypical antipsychotics or second-generation antipsychotics. A brand name is Geodon®. I take ziprasidone. Can it make it harder for me to get pregnant? A reported side effect of ziprasidone is sexual dysfunction (problems with sexual desire, sexual arousal, orgasms, or sexual pain disorders). If a person has sexual dysfunction, it might make it harder for to get pregnant. I just found out that I am pregnant. Should I stop taking ziprasidone? Talk with your healthcare providers before making any changes to how you take this medication. For some people, the benefits of staying on an antipsychotic medication during pregnancy can outweigh any potential concerns. Does taking ziprasidone increase the chance for miscarriage? Miscarriage can occur in any pregnancy. It is not known if taking ziprasidone would increase this chance for miscarriage. There are no well controlled studies on ziprasidone use during pregnancy in humans. One prescription- based study saw a slightly higher chance of a miscarriage among people who were pregnant and filled a prescription for ziprasidone in pregnancy. This type of study cannot tell us if the person actually used the medication. In addition, since untreated or uncontrolled mood disorders can increase the chance of miscarriage, it might have been the pregnant person’s condition that led to the miscarriage. -

Paper Clip—High Risk Medications
You belong. PAPER CLIP—HIGH RISK MEDICATIONS Description: Some medications may be risky for people over 65 years of age and there may be safer drug choices to treat some conditions. It is recommended that medications are reviewed regularly with your providers/prescriber to ensure patient safety. Condition Treated Current Medication(s)-High Risk Safer Alternatives to Consider Urinary Tract Infections (UTIs), Nitrofurantoin Bactrim (sulfamethoxazole/trimethoprim) or recurrent UTIs, or Prophylaxis (Macrobid, or Macrodantin) Trimpex/Proloprim/Primsol (trimethoprim) Allergies Hydroxyzine (Vistaril, Atarax) Xyzal (Levocetirizine) Anxiety Hydroxyzine (Vistaril, Atarax) Buspar (Buspirone), Paxil (Paroxetine), Effexor (Venlafaxine) Parkinson’s Hydroxyzine (Vistaril, Atarax) Symmetrel (Amantadine), Sinemet (Carbidopa/levodopa), Selegiline (Eldepryl) Motion Sickness Hydroxyzine (Vistaril, Atarax) Antivert (Meclizine) Nausea & Vomiting Hydroxyzine (Vistaril, Atarax) Zofran (Ondansetron) Insomnia Hydroxyzine (Vistaril, Atarax) Ramelteon (Rozerem), Doxepin (Silenor) Chronic Insomnia Eszopiclone (Lunesta) Ramelteon (Rozerem), Doxepin (Silenor) Chronic Insomnia Zolpidem (Ambien) Ramelteon (Rozerem), Doxepin (Silenor) Chronic Insomnia Zaleplon (Sonata) Ramelteon (Rozerem), Doxepin (Silenor) Muscle Relaxants Cyclobenazprine (Flexeril, Amrix) NSAIDs (Ibuprofen, naproxen) or Hydrocodone Codeine Tramadol (Ultram) Baclofen (Lioresal) Tizanidine (Zanaflex) Migraine Prophylaxis Elavil (Amitriptyline) Propranolol (Inderal), Timolol (Blocadren), Topiramate (Topamax), -

Psychedelics in Psychiatry: Neuroplastic, Immunomodulatory, and Neurotransmitter Mechanismss
Supplemental Material can be found at: /content/suppl/2020/12/18/73.1.202.DC1.html 1521-0081/73/1/202–277$35.00 https://doi.org/10.1124/pharmrev.120.000056 PHARMACOLOGICAL REVIEWS Pharmacol Rev 73:202–277, January 2021 Copyright © 2020 by The Author(s) This is an open access article distributed under the CC BY-NC Attribution 4.0 International license. ASSOCIATE EDITOR: MICHAEL NADER Psychedelics in Psychiatry: Neuroplastic, Immunomodulatory, and Neurotransmitter Mechanismss Antonio Inserra, Danilo De Gregorio, and Gabriella Gobbi Neurobiological Psychiatry Unit, Department of Psychiatry, McGill University, Montreal, Quebec, Canada Abstract ...................................................................................205 Significance Statement. ..................................................................205 I. Introduction . ..............................................................................205 A. Review Outline ........................................................................205 B. Psychiatric Disorders and the Need for Novel Pharmacotherapies .......................206 C. Psychedelic Compounds as Novel Therapeutics in Psychiatry: Overview and Comparison with Current Available Treatments . .....................................206 D. Classical or Serotonergic Psychedelics versus Nonclassical Psychedelics: Definition ......208 Downloaded from E. Dissociative Anesthetics................................................................209 F. Empathogens-Entactogens . ............................................................209 -

Electrocardiographic Changes in Children Treated with Ziprasidone : a Prospective Study and Review Jennifer Melissa Blair Yale University
Yale University EliScholar – A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine 2004 Electrocardiographic changes in children treated with ziprasidone : a prospective study and review Jennifer Melissa Blair Yale University Follow this and additional works at: http://elischolar.library.yale.edu/ymtdl Recommended Citation Blair, Jennifer Melissa, "Electrocardiographic changes in children treated with ziprasidone : a prospective study and review" (2004). Yale Medicine Thesis Digital Library. 2405. http://elischolar.library.yale.edu/ymtdl/2405 This Open Access Thesis is brought to you for free and open access by the School of Medicine at EliScholar – A Digital Platform for Scholarly Publishing at Yale. It has been accepted for inclusion in Yale Medicine Thesis Digital Library by an authorized administrator of EliScholar – A Digital Platform for Scholarly Publishing at Yale. For more information, please contact [email protected]. YALE UNIVERSITY CUSHING/WHITNEY MEDICAL LIBRARY Permission to photocopy or microfilm processing of this thesis for the purpose of individual scholarly consultation or reference is hereby granted by the author. This permission is not to be interpreted as affecting publication of this work or otherwise placing it in the public domain, and the author reserves all rights of ownership guaranteed under common law protection of unpublished manuscripts. it Date Digitized by the Internet Archive in 2017 with funding from The National Endowment for the Humanities and the Arcadia Fund https://archive.org/details/electrocardiograOOblai ELECTROCARDIOGRAPHIC CHANGES IN CHILDREN TREATED WITH ZIPRASIDONE: A PROSPECTIVE STUDY AND REVIEW A Thesis Submitted to the Yale University School of Medicine in Partial Fulfillment of the Requirements for the Degree of Doctor of Medicine by Jennifer Melissa Blair 2004 r //-i * Y/i- 701Y ELECTROCARDIOGRAPHIC CHANGES IN CHILDREN TREATED WITH ZIPRASIDONE: A PROSPECTIVE STUDY AND REVIEW Jennifer Blair, Lawrence Scahill, Matthew State, and Andres Martin. -

Pharmacogenetic Studies Investigating the Adverse Effects of Antipsychotics
online © ML Comm 0REVIEW ARTICLE0 Psychiatry Investig 2007;4:66-75 Print ISSN 1738-3684 / On-line ISSN 1976-3026 Pharmacogenetic Studies Investigating the Adverse Effects of Antipsychotics Heon-Jeong Lee, MD, PhD The pharmacogenetic study of antipsychotics has been developed along with the develop- Department of Psychiatry, ment of general techniques of genetic analysis. Because there are no significant differences Division of Brain Korea in the clinical efficacy of the various antipsychotics, it is important to prevent the adverse 21 for Biomedical Science, Korea University College of Medicine, effects of antipsychotics. Therefore, pharmacogenetic studies concerning antipsychotics have Seoul, Korea been primarily focused on their adverse effects. The most significant finding of the previous studies is the association between drug effects and drug metabolic polymorphisms, mainly in the cytochrome P450 (CYP) genes. Patients with genetically determined to be CYP poor metabolizers (PMs) may require lower doses of antipsychotic medications. On the other hand, CYP ultrarapid matabolizers (UMs) will need an increased dosage in order to obtain a therapeutic response. Genetic variations in the dopamine and serotonin receptor genes have been reported to be associated with the adverse effects of antipsychotics, reflecting the affinities that most antipsychotics have for these receptors. In particular, there is evidence to suggest an association between dopamine 2 receptor polymorphisms and a dopamine 3 receptor polymorphism and antipsychotic-induced tardive dyskinesia. Several studies were recently performed to determine the genetic susceptibility to antipsychotic-induced weight gain and metabolic syndrome. Adrenergic 2a receptor, leptin gene, and serotonin 2C receptor gene variants have been reported to be associated with drug-induced weight gain. -

CENTRAL NERVOUS SYSTEM DEPRESSANTS Opioid Pain Relievers Anxiolytics (Also Belong to Psychiatric Medication Category) • Codeine (In 222® Tablets, Tylenol® No
CENTRAL NERVOUS SYSTEM DEPRESSANTS Opioid Pain Relievers Anxiolytics (also belong to psychiatric medication category) • codeine (in 222® Tablets, Tylenol® No. 1/2/3/4, Fiorinal® C, Benzodiazepines Codeine Contin, etc.) • heroin • alprazolam (Xanax®) • hydrocodone (Hycodan®, etc.) • chlordiazepoxide (Librium®) • hydromorphone (Dilaudid®) • clonazepam (Rivotril®) • methadone • diazepam (Valium®) • morphine (MS Contin®, M-Eslon®, Kadian®, Statex®, etc.) • flurazepam (Dalmane®) • oxycodone (in Oxycocet®, Percocet®, Percodan®, OxyContin®, etc.) • lorazepam (Ativan®) • pentazocine (Talwin®) • nitrazepam (Mogadon®) • oxazepam ( Serax®) Alcohol • temazepam (Restoril®) Inhalants Barbiturates • gases (e.g. nitrous oxide, “laughing gas”, chloroform, halothane, • butalbital (in Fiorinal®) ether) • secobarbital (Seconal®) • volatile solvents (benzene, toluene, xylene, acetone, naptha and hexane) Buspirone (Buspar®) • nitrites (amyl nitrite, butyl nitrite and cyclohexyl nitrite – also known as “poppers”) Non-Benzodiazepine Hypnotics (also belong to psychiatric medication category) • chloral hydrate • zopiclone (Imovane®) Other • GHB (gamma-hydroxybutyrate) • Rohypnol (flunitrazepam) CENTRAL NERVOUS SYSTEM STIMULANTS Amphetamines Caffeine • dextroamphetamine (Dexadrine®) Methelynedioxyamphetamine (MDA) • methamphetamine (“Crystal meth”) (also has hallucinogenic actions) • methylphenidate (Biphentin®, Concerta®, Ritalin®) • mixed amphetamine salts (Adderall XR®) 3,4-Methelynedioxymethamphetamine (MDMA, Ecstasy) (also has hallucinogenic actions) Cocaine/Crack