Paper Clip—High Risk Medications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Journal of Pharmacology and Experimental Therapeutics
Journal of Pharmacology and Experimental Therapeutics Molecular Determinants of Ligand Selectivity for the Human Multidrug And Toxin Extrusion Proteins, MATE1 and MATE-2K Bethzaida Astorga, Sean Ekins, Mark Morales and Stephen H Wright Department of Physiology, University of Arizona, Tucson, AZ 85724, USA (B.A., M.M., and S.H.W.) Collaborations in Chemistry, 5616 Hilltop Needmore Road, Fuquay-Varina NC 27526, USA (S.E.) Supplemental Table 1. Compounds selected by the common features pharmacophore after searching a database of 2690 FDA approved compounds (www.collaborativedrug.com). FitValue Common Name Indication 3.93897 PYRIMETHAMINE Antimalarial 3.3167 naloxone Antidote Naloxone Hydrochloride 3.27622 DEXMEDETOMIDINE Anxiolytic 3.2407 Chlordantoin Antifungal 3.1776 NALORPHINE Antidote Nalorphine Hydrochloride 3.15108 Perfosfamide Antineoplastic 3.11759 Cinchonidine Sulfate Antimalarial Cinchonidine 3.10352 Cinchonine Sulfate Antimalarial Cinchonine 3.07469 METHOHEXITAL Anesthetic 3.06799 PROGUANIL Antimalarial PROGUANIL HYDROCHLORIDE 100MG 3.05018 TOPIRAMATE Anticonvulsant 3.04366 MIDODRINE Antihypotensive Midodrine Hydrochloride 2.98558 Chlorbetamide Antiamebic 2.98463 TRIMETHOPRIM Antibiotic Antibacterial 2.98457 ZILEUTON Antiinflammatory 2.94205 AMINOMETRADINE Diuretic 2.89284 SCOPOLAMINE Antispasmodic ScopolamineHydrobromide 2.88791 ARTICAINE Anesthetic 2.84534 RITODRINE Tocolytic 2.82357 MITOBRONITOL Antineoplastic Mitolactol 2.81033 LORAZEPAM Anxiolytic 2.74943 ETHOHEXADIOL Insecticide 2.64902 METHOXAMINE Antihypotensive Methoxamine -

NH Healthy Families Preferred Drug List (PDL) Is the List of Covered Drugs
Pharmacy Program NH Healthy Families is committed to providing appropriate, high quality, and cost effective drug therapy to all NH Healthy Families members. NH Healthy Families works with providers and pharmacists to ensure that medications used to treat a variety of conditions and diseases are covered. NH Healthy Families covers prescription medications and certain over-the-counter (OTC) medications when ordered by a practitioner. The pharmacy program does not cover all medications. Some medications require prior authorization (PA) or have limitations on age, dosage, and maximum quantities. Preferred Drug List The NH Healthy Families Preferred Drug List (PDL) is the list of covered drugs. The PDL applies to drugs members can receive at retail pharmacies. The NH Healthy Families PDL is continually evaluated by the NH Healthy Families Pharmacy and Therapeutics (P&T) Committee to promote the appropriate and cost- effective use of medications. The Committee is composed of the NH Healthy Families Medical Director, NH Healthy Families Pharmacy Director, and several New Hampshire physicians, pharmacists, and other healthcare professionals. Pharmacy Benefit Manager NH Healthy Families works with Envolve Pharmacy Solutions to process pharmacy claims for prescribed drugs. Some drugs on the NH Healthy Families PDL may require PA, and Envolve Pharmacy Solutions is responsible for administering this process. Envolve Pharmacy Solutions is our Pharmacy Benefit Manager (PBM). Specialty Drugs NH Healthy Families contracts with a number of specialty pharmacies, such as AcariaHealth Specialty Pharmacy, to ensure members have adequate access to the specialty drugs they require. Specialty drugs, such as biopharmaceuticals and injectables, may require PA to be approved for payment by NH Healthy Families. -
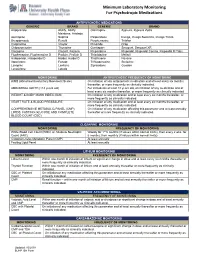
Minimum Laboratory Monitoring for Psychotropic Medications
Minimum Laboratory Monitoring For Psychotropic Medications ANTIPSYCHOTIC MEDICATIONS GENERIC BRAND GENERIC BRAND Aripiprazole Abilify, Abilify Olanzapine Zyprexa, Zyprexa Zydis Maintena, Aristada Asenapine Saphris Paliperidone Invega, Invega Sustenna, Invega Trinza Brexpiprazole Rexulti Perphenazine Trilafon Cariprazine Vraylar Pimozide Orap Chlorpromazine Thorazine Quetiapine Seroquel, Seroquel XR Clozapine Clozaril, Fazaclo Risperidone Risperdal, Risperdal Consta, Risperdal M Tabs Fluphenazine, Fluphenazine D Prolixin, Prolixin D Thioridazine Mellaril Haloperidol, Haloperidol D Haldol, Haldol D Thiothixene Navane Iloperidone Fanapt Trifluoperazine Stelazine Loxapine Loxitane Ziprasidone Geodon Lurasidone Latuda MONITORING ANTIPSYCHOTIC FREQUENCY OF MONITORING AIMS (Abnormal Involuntary Movement Scale) On initiation of any antipsychotic medication and at least every six months thereafter, or more frequently as clinically indicated. ABDOMINAL GIRTH (>18 years old) For individuals at least 18 years old, on initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. WEIGHT & BODY MASS INDEX (BMI) On initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. HEART RATE & BLOOD PRESSSURE On initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. COMPREHENSIVE METABOLIC PANEL (CMP), On initiation of any medication affecting this parameter and at least annually LIPIDS, FASTING -

Schizophrenia Care Guide
August 2015 CCHCS/DHCS Care Guide: Schizophrenia SUMMARY DECISION SUPPORT PATIENT EDUCATION/SELF MANAGEMENT GOALS ALERTS Minimize frequency and severity of psychotic episodes Suicidal ideation or gestures Encourage medication adherence Abnormal movements Manage medication side effects Delusions Monitor as clinically appropriate Neuroleptic Malignant Syndrome Danger to self or others DIAGNOSTIC CRITERIA/EVALUATION (PER DSM V) 1. Rule out delirium or other medical illnesses mimicking schizophrenia (see page 5), medications or drugs of abuse causing psychosis (see page 6), other mental illness causes of psychosis, e.g., Bipolar Mania or Depression, Major Depression, PTSD, borderline personality disorder (see page 4). Ideas in patients (even odd ideas) that we disagree with can be learned and are therefore not necessarily signs of schizophrenia. Schizophrenia is a world-wide phenomenon that can occur in cultures with widely differing ideas. 2. Diagnosis is made based on the following: (Criteria A and B must be met) A. Two of the following symptoms/signs must be present over much of at least one month (unless treated), with a significant impact on social or occupational functioning, over at least a 6-month period of time: Delusions, Hallucinations, Disorganized Speech, Negative symptoms (social withdrawal, poverty of thought, etc.), severely disorganized or catatonic behavior. B. At least one of the symptoms/signs should be Delusions, Hallucinations, or Disorganized Speech. TREATMENT OPTIONS MEDICATIONS Informed consent for psychotropic -

Medicines That Affect Fluid Balance in the Body
the bulk of stools by getting them to retain liquid, which encourages the Medicines that affect fluid bowels to push them out. balance in the body Osmotic laxatives e.g. Lactulose, Macrogol - these soften stools by increasing the amount of water released into the bowels, making them easier to pass. Older people are at higher risk of dehydration due to body changes in the ageing process. The risk of dehydration can be increased further when Stimulant laxatives e.g. Senna, Bisacodyl - these stimulate the bowels elderly patients are prescribed medicines for chronic conditions due to old speeding up bowel movements and so less water is absorbed from the age. stool as it passes through the bowels. Some medicines can affect fluid balance in the body and this may result in more water being lost through the kidneys as urine. Stool softener laxatives e.g. Docusate - These can cause more water to The medicines that can increase risk of dehydration are be reabsorbed from the bowel, making the stools softer. listed below. ANTACIDS Antacids are also known to cause dehydration because of the moisture DIURETICS they require when being absorbed by your body. Drinking plenty of water Diuretics are sometimes called 'water tablets' because they can cause you can reduce the dry mouth, stomach cramps and dry skin that is sometimes to pass more urine than usual. They work on the kidneys by increasing the associated with antacids. amount of salt and water that comes out through the urine. Diuretics are often prescribed for heart failure patients and sometimes for patients with The major side effect of antacids containing magnesium is diarrhoea and high blood pressure. -

Drugs to Avoid in Patients with Dementia
Detail-Document #240510 -This Detail-Document accompanies the related article published in- PHARMACIST’S LETTER / PRESCRIBER’S LETTER May 2008 ~ Volume 24 ~ Number 240510 Drugs To Avoid in Patients with Dementia Elderly people with dementia often tolerate drugs less favorably than healthy older adults. Reasons include increased sensitivity to certain side effects, difficulty with adhering to drug regimens, and decreased ability to recognize and report adverse events. Elderly adults with dementia are also more prone than healthy older persons to develop drug-induced cognitive impairment.1 Medications with strong anticholinergic (AC) side effects, such as sedating antihistamines, are well- known for causing acute cognitive impairment in people with dementia.1-3 Anticholinergic-like effects, such as urinary retention and dry mouth, have also been identified in drugs not typically associated with major AC side effects (e.g., narcotics, benzodiazepines).3 These drugs are also important causes of acute confusional states. Factors that may determine whether a patient will develop cognitive impairment when exposed to ACs include: 1) total AC load (determined by number of AC drugs and dose of agents utilized), 2) baseline cognitive function, and 3) individual patient pharmacodynamic and pharmacokinetic features (e.g., renal/hepatic function).1 Evidence suggests that impairment of cholinergic transmission plays a key role in the development of Alzheimer’s dementia. Thus, the development of the cholinesterase inhibitors (CIs). When used appropriately, the CIs (donepezil [Aricept], rivastigmine [Exelon], and galantamine [Razadyne, Reminyl in Canada]) may slow the decline of cognitive and functional impairment in people with dementia. In order to achieve maximum therapeutic effect, they ideally should not be used in combination with ACs, agents known to have an opposing mechanism of action.1,2 Roe et al studied AC use in 836 elderly patients.1 Use of ACs was found to be greater in patients with probable dementia than healthy older adults (33% vs. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Adverse Reactions to Hallucinogenic Drugs. 1Rnstttutton National Test
DOCUMENT RESUME ED 034 696 SE 007 743 AUTROP Meyer, Roger E. , Fd. TITLE Adverse Reactions to Hallucinogenic Drugs. 1rNSTTTUTTON National Test. of Mental Health (DHEW), Bethesda, Md. PUB DATP Sep 67 NOTE 118p.; Conference held at the National Institute of Mental Health, Chevy Chase, Maryland, September 29, 1967 AVATLABLE FROM Superintendent of Documents, Government Printing Office, Washington, D. C. 20402 ($1.25). FDPS PRICE FDPS Price MFc0.50 HC Not Available from EDRS. DESCPTPTOPS Conference Reports, *Drug Abuse, Health Education, *Lysergic Acid Diethylamide, *Medical Research, *Mental Health IDENTIFIEPS Hallucinogenic Drugs ABSTPACT This reports a conference of psychologists, psychiatrists, geneticists and others concerned with the biological and psychological effects of lysergic acid diethylamide and other hallucinogenic drugs. Clinical data are presented on adverse drug reactions. The difficulty of determining the causes of adverse reactions is discussed, as are different methods of therapy. Data are also presented on the psychological and physiolcgical effects of L.S.D. given as a treatment under controlled medical conditions. Possible genetic effects of L.S.D. and other drugs are discussed on the basis of data from laboratory animals and humans. Also discussed are needs for futher research. The necessity to aviod scare techniques in disseminating information about drugs is emphasized. An aprentlix includes seven background papers reprinted from professional journals, and a bibliography of current articles on the possible genetic effects of drugs. (EB) National Clearinghouse for Mental Health Information VA-w. Alb alb !bAm I.S. MOMS Of NAM MON tMAN IONE Of NMI 105 NUNN NU IN WINES UAWAS RCM NIN 01 NUN N ONMININI 01011110 0. -

Tizanidine Brands: Zanaflex®
Medication Information Sheet Tizanidine brands: Zanaflex® Medications are only ONE part of a successful treatment plan. They are appropriate when they provide benefit, improve function and have either no or mild, manageable side effects. Importantly, medications (even if natural) are chemical substances not expected in the body, and as such have side effects. Some of the side effects might be unknown. The use of medications/drugs for any purpose requires patient consent. This practice does NOT require a patient to use any medication. Information & potential benefits Tizanidine is a medication that helps with muscle spasms and musculoskeletal pain syndromes; there is evidence that it helps neuropathic and musculoskeletal pain through alpha‐2‐receptor activity. Studies have shown Zanaflex helpful for neuropathic pain and some types of headache. It is currently FDA approved for muscle spasticity. Potential risks and side effects Tizanidine should be used carefully in cases of liver or kidney disease, low blood pressure, or heart conduction problems (QT interval problems). It should not be used with Luvox or with the antibiotic Cipro (ciprofloxacin). There are many other drugs that can interact with Tizanadine. In addition to the standard side effects listed in the disclaimer, common side effects or Zanaflex include dry mouth, sleepiness, dizziness, asthenia, infection, constipation, urinary frequency, flu‐like feeling, low blood pressure, more spasms, sore throat and runny nose. More serious side effects include liver damage, severe slowing of the heart beat and hallucinations. Tizanidine occasionally causes liver injury. In controlled clinical studies, approximately 5% of patients treated with tizanidine had non‐serious elevations of liver function tests. -

Current P SYCHIATRY
Current p SYCHIATRY N ew Investigators Tips to manage and prevent discontinuation syndromes Informed tapering can protect patients when you stop a medication Sriram Ramaswamy, MD Shruti Malik, MBBS, MHSA Vijay Dewan, MD Instructor, department of psychiatry Foreign medical graduate Assistant professor Creighton University Department of psychiatry Omaha, NE University of Nebraska Medical Center Omaha, NE bruptly stopping common psychotropics New insights on psychotropic A —particularly antidepressants, benzodi- drug safety and side effects azepines, or atypical antipsychotics—can trigger a discontinuation syndrome, with: This paper was among those entered in the 2005 • rebound or relapse of original symptoms Promising New Investigators competition sponsored • uncomfortable new physical and psycho- by the Neuroleptic Malignant Syndrome Information Service (NMSIS). The theme of this year’s competition logical symptoms was “New insights on psychotropic drug safety and • physiologic withdrawal at times. side effects.” To increase health professionals’ awareness of URRENT SYCHIATRY 1 C P is honored to publish this peer- the risk of these adverse effects, this article reviewed, evidence-based article on a clinically describes discontinuation syndromes associated important topic for practicing psychiatrists. with various psychotropics and offers strategies to NMSIS is dedicated to reducing morbidity and anticipate, recognize, and manage them. mortality of NMS by improving medical and psychiatric care of patients with heat-related disorders; providing -

Clinical Review, Adverse Events
Clinical Review, Adverse Events Drug: Carbamazepine NDA: 16-608, Tegretol 20-712, Carbatrol 21-710, Equetro Adverse Event: Stevens-Johnson Syndrome Reviewer: Ronald Farkas, MD, PhD Medical Reviewer, DNP, ODE I 1. Executive Summary 1.1 Background Carbamazepine (CBZ) is an anticonvulsant with FDA-approved indications in epilepsy, bipolar disorder and neuropathic pain. CBZ is associated with Stevens-Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN), closely related serious cutaneous adverse drug reactions that can be permanently disabling or fatal. Other anticonvulsants, including phenytoin, phenobarbital, and lamotrigine are also associated with SJS/TEN, as are members of a variety of other drug classes, including nonsteriodal anti-inflammatory drugs and sulfa drugs. The incidence of CBZ-associated SJS/TEN has been considered “extremely rare,” as noted in current U.S. drug labeling. However, recent publications and postmarketing data suggest that CBZ- associated SJS/TEN occurs at a much higher rate in some Asian populations, about 2.5 cases per 1,000 new exposures, and that most of this increased risk is in individuals carrying a specific human leukocyte antigen (HLA) allele, HLA-B*1502. This HLA-B allele is present in about 5- to 20% of many, but not all, Asian populations, and is also present in about 2- to 4% of South Asians/Indians. The allele is also present at a lower frequency, < 1%, in several other ethnic groups around the world (although likely due to distant Asian ancestry). About 10% of U.S. Asians carry HLA-B*1502. HLA-B*1502 is generally not present in the U.S. -
Hydroxyzine Prescribing Information
Hydroxyzine Hydroxyzine Hydrochloride Hydrochloride Injection, USP Injection, USP (For Intramuscular Use Only) (For Intramuscular Use Only) Rx Only Rx Only DESCRIPTION: Hydroxyzine hydrochloride has the chemical name of (±)-2-[2-[4-( p-Chloro- a- phenylbenzyl)-1-piperazinyl]ethoxy]ethanol dihydrochloride and occurs as a white, odorless powder which is very soluble in water. It has the following structural formula: Molecular Formula: C 21 H27 ClN 2O2•2HCl Molecular Weight: 447.83 Hydroxyzine Hydrochloride Injection, USP is a sterile aqueous solution intended for intramuscular administration. Each mL contains: Hydroxyzine HCl 25 mg or 50 mg, Benzyl Alcohol 0.9%, and Water for Injection q.s. pH adjusted with Sodium Hydroxide and/or Hydrochloric Acid. CLINICAL PHARMACOLOGY: Hydroxyzine hydrochloride is unrelated chemically to phenothiazine, reserpine, and meprobamate. Hydroxyzine has demonstrated its clinical effectiveness in the chemotherapeutic aspect of the total management of neuroses and emotional disturbances manifested by anxiety, tension, agitation, apprehension or confusion. Hydroxyzine has been shown clinically to be a rapid-acting true ataraxic with a wide margin of safety. It induces a calming effect in anxious, tense, psychoneurotic adults and also in anxious, hyperkinetic children without impairing mental alertness. It is not a cortical depressant, but its action may be due to a suppression of activity in certain key regions of the subcortical area of the central nervous system. Primary skeletal muscle relaxation has been demonstrated experimentally. Hydroxyzine has been shown experimentally to have antispasmodic properties, apparently mediated through interference with the mechanism that responds to spasmogenic agents such as serotonin, acetylcholine, and histamine. Antihistaminic effects have been demonstrated experimentally and confirmed clinically.