Restoration of Noosa Estuary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Known Impacts of Tropical Cyclones, East Coast, 1858 – 2008 by Mr Jeff Callaghan Retired Senior Severe Weather Forecaster, Bureau of Meteorology, Brisbane
ARCHIVE: Known Impacts of Tropical Cyclones, East Coast, 1858 – 2008 By Mr Jeff Callaghan Retired Senior Severe Weather Forecaster, Bureau of Meteorology, Brisbane The date of the cyclone refers to the day of landfall or the day of the major impact if it is not a cyclone making landfall from the Coral Sea. The first number after the date is the Southern Oscillation Index (SOI) for that month followed by the three month running mean of the SOI centred on that month. This is followed by information on the equatorial eastern Pacific sea surface temperatures where: W means a warm episode i.e. sea surface temperature (SST) was above normal; C means a cool episode and Av means average SST Date Impact January 1858 From the Sydney Morning Herald 26/2/1866: an article featuring a cruise inside the Barrier Reef describes an expedition’s stay at Green Island near Cairns. “The wind throughout our stay was principally from the south-east, but in January we had two or three hard blows from the N to NW with rain; one gale uprooted some of the trees and wrung the heads off others. The sea also rose one night very high, nearly covering the island, leaving but a small spot of about twenty feet square free of water.” Middle to late Feb A tropical cyclone (TC) brought damaging winds and seas to region between Rockhampton and 1863 Hervey Bay. Houses unroofed in several centres with many trees blown down. Ketch driven onto rocks near Rockhampton. Severe erosion along shores of Hervey Bay with 10 metres lost to sea along a 32 km stretch of the coast. -

Restricted Water Ski Areas in Queensland
Restricted Water Ski areas in Queensland Watercourse Date of Gazettal Any person operating a ship towing anyone by a line attached to the ship (including for example a person water skiing or riding on a toboggan or tube) within the waters listed below endangers marine safety. Brisbane River 20/10/2006 South Brisbane and Town Reaches of the Brisbane River between the Merivale Bridge and the Story Bridge. Burdekin River, Charters Towers 13/09/2019 All waters of The Weir on the Burdekin River, Charters Towers. Except: • commencing at a point on the waterline of the eastern bank of the Burdekin River nearest to location 19°55.279’S, 146°16.639’E, • then generally southerly along the waterline of the eastern bank to a point nearest to location 19°56.530’S, 146°17.276’E, • then westerly across Burdekin River to a point on the waterline of the western bank nearest to location 19°56.600’S, 146°17.164’E, • then generally northerly along the waterline of the western bank to a point on the waterline nearest to location 19°55.280’S, 146°16.525’E, • then easterly across the Burdekin River to the point of commencement. As shown on the map S8sp-73 prepared by Maritime Safety Queensland (MSQ) which can be found on the MSQ website at www.msq.qld.gov.au/s8sp73map and is held at MSQ’s Townsville Office. Burrum River .12/07/1996 The waters of the Burrum River within 200 metres north from the High Water mark of the southern river bank and commencing at a point 50 metres downstream of the public boat ramp off Burrum Heads Road to a point 200 metres upstream of the upstream boundary of Lions Park, Burrum Heads. -

Beacon to Beacon Guide: Noosa River
Maritime Safety Queensland Noosa River Boat Ramp, Tewantin Beacon to Beacon Guide Noosa River Published by For commercial use terms and conditions Maritime Safety Queensland Please visit the Maritime Safety Queensland website at www.msq.qld.gov.au © Copyright The State of Queensland (Department of Transport and Main Roads) 2021 ‘How to’ use this guide Use this Beacon to Beacon Guide with To view a copy of this licence, visit the ‘How to’ and legend booklet available from https://creativecommons.org/licenses/by/4.0/ www.msq.qld.gov.au Noosa River Cooloola Beach Key Sheet Next series NOOSA Great Sandy Strait RIVER Marine rescue services 10 CG Noosa Enlargements A Boreen Point B Noosa Marina Lake Cooloola Shark control apparatus Lake exclusion zones Como Exclusion zones exist for waters within 20 metres of any shark control apparatus. It is an offence (fines may apply) to be in an exclusion zone if not transiting directly through. For further information see the Department of Agriculture and Fisheries website at www.daf.qld.gov.au. Teewah Beach Depth contour date information Elanda Point Depth contours shown on the maps within this guide were surveyed by Maritime Safety Queensland 2000-2016. Kin Kin Some 0m contours have been approximated from recent Lake Teewah Cootharaba TMR air photography. A Boreen Point SOUTH CORAL NOOSA SEA RIVER PACIFIC Lake Cooroibah Six Mile Creek Dam Noosa Head TEWANTIN 10 Lake 10 Macdonald B NOOSA HEADS OCEAN Sunshine Cooroy Beach For maps and information on the Noosa River marine zones please visit Maritime Safety Queensland website (www.msq.qld.gov.au) under the Waterways tab and click on Marine Zones. -

Land Cover Change in the South East Queensland Catchments Natural Resource Management Region 2010–11
Department of Science, Information Technology, Innovation and the Arts Land cover change in the South East Queensland Catchments Natural Resource Management region 2010–11 Summary The woody vegetation clearing rate for the SEQ region for 10 2010–11 dropped to 3193 hectares per year (ha/yr). This 9 8 represented a 14 per cent decline from the previous era. ha/year) 7 Clearing rates of remnant woody vegetation decreased in 6 5 2010-11 to 758 ha/yr, 33 per cent lower than the previous era. 4 The replacement land cover class of forestry increased by 3 2 a further 5 per cent over the previous era and represented 1 Clearing Rate (,000 26 per cent of the total woody vegetation 0 clearing rate in the region. Pasture 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 remained the dominant replacement All Woody Clearing Woody Remnant Clearing land cover class at 34 per cent of total clearing. Figure 1. Woody vegetation clearing rates in the South East Queensland Catchments NRM region. Figure 2. Woody vegetation clearing for each change period. Great state. Great opportunity. Woody vegetation clearing by Woody vegetation clearing by remnant status tenure Table 1. Remnant and non-remnant woody vegetation clearing Table 2. Woody vegetation clearing rates in the South East rates in the South East Queensland Catchments NRM region. Queensland Catchments NRM region by tenure. Woody vegetation clearing rate (,000 ha/yr) of Woody vegetation clearing rate (,000 ha/yr) on Non-remnant Remnant -

22Nd March 2019
This booklet has been prepared to commemorate the historic 200th General Meeting of the Mary River Catchment Coordinating Committee on Friday 22nd of March 2019 at Garapine; the location of the inaugural meeting in November 1993. It adds to a previous booklet prepared for the 100th meeting which was held at the Gympie Civic Centre on Wednesday 16th February 2005. For almost 25 years, the MRCCC has forged productive partnerships with thousands of stakeholders throughout the Mary River catchment and beyond; government at all three levels, industry, farmers, large and small rural and urban landholders, landcare and environment groups, recreational and commercial fishing interests, forestry, irrigators, Waterwatch volunteers, researchers, school students, and particularly the long-running working partnership with the Gympie District Beef Liaison Group. These partnerships have triggered a phenomenal groundswell of interest and activities in natural resource management across the Mary River catchment. The wider community is beginning to understand many of the causes of environmental degradation. The farming community is embracing sustainable production as a means of increasing productivity whilst protecting natural assets. Governments at all levels now recognise that community engagement is critical to environmental repair and ecological protection. Triple bottom line objectives are now commonplace in strategic planning documents. So what were the factors that led to the need for an “across the board” shift in philosophy? In the 1990’s, the Mary River was described as one of the most degraded catchments in Queensland. European settlement resulted in extensive clearing of the riverbanks. In recent times, massive land use change due to subdivision, population pressure and other factors together with increasing demand for water resources led to deteriorating catchment condition. -

Fisheries Guidelines for Design of Stream Crossings
Fish Habitat Guideline FHG 001 FISH PASSAGE IN STREAMS Fisheries guidelines for design of stream crossings Elizabeth Cotterell August 1998 Fisheries Group DPI ISSN 1441-1652 Agdex 486/042 FHG 001 First published August 1998 Information contained in this publication is provided as general advice only. For application to specific circumstances, professional advice should be sought. The Queensland Department of Primary Industries has taken all reasonable steps to ensure the information contained in this publication is accurate at the time of publication. Readers should ensure that they make appropriate enquiries to determine whether new information is available on the particular subject matter. © The State of Queensland, Department of Primary Industries 1998 Copyright protects this publication. Except for purposes permitted by the Copyright Act, reproduction by whatever means is prohibited without the prior written permission of the Department of Primary Industries, Queensland. Enquiries should be addressed to: Manager Publishing Services Queensland Department of Primary Industries GPO Box 46 Brisbane QLD 4001 Fisheries Guidelines for Design of Stream Crossings BACKGROUND Introduction Fish move widely in rivers and creeks throughout Queensland and Australia. Fish movement is usually associated with reproduction, feeding, escaping predators or dispersing to new habitats. This occurs between marine and freshwater habitats, and wholly within freshwater. Obstacles to this movement, such as stream crossings, can severely deplete fish populations, including recreational and commercial species such as barramundi, mullet, Mary River cod, silver perch, golden perch, sooty grunter and Australian bass. Many Queensland streams are ephemeral (they may flow only during the wet season), and therefore crossings must be designed for both flood and drought conditions. -

Feedback on Draft Noosa River Plan Version 1
General Committee Agenda 18 January 2021 Attachment 2 to Item 2 – Consultation Report River Plan Version 1 (2018) Feedback on Draft Noosa River Plan version 1 The information below provides a collation of detailed feedback on the Draft Noosa River Plan received from the community during the public consultation period from 20 July to 26 August 2018. This feedback consists of responses to the Noosa River Plan SURVEY published on Council’s Your Say Noosa (YSN) website, and written submissions received from individuals and organisations. Three questions were posed in the SURVEY: 1. What do you think will be most important in maintaining and improving the quality of the Noosa River system? 2. Are there any actions to consider for inclusion in the Draft Noosa River Plan? 3. The cost of implementing the actions recommended in the Draft Noosa River Plan is estimated to be $2.23M over 5 years (i.e. $446,000 pa). Are you willing to pay extra (via General Rates or Environment Levy and/or Tourism and Economic Levy) for Noosa Council to fully implement the River Plan so that the Noosa River has a better chance of remaining a pristine and vital community asset? If yes, how much per year ($5, $10, $15, $20)? Summary of feedback The top four (4) priorities deemed most important to maintain and improve the quality of the Noosa River system were identified as: • managing pollution and litter running into the river • protecting wetlands, riparian and coastal areas • reducing sediment from upstream entering the river • controlling recreational use of the -
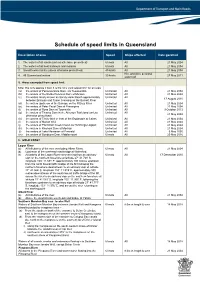
Schedule of Speed Limits in Queensland
Schedule of speed limits in Queensland Description of area Speed Ships affected Date gazetted 1. The waters of all canals (unless otherwise prescribed) 6 knots All 21 May 2004 2. The waters of all boat harbours and marinas 6 knots All 21 May 2004 3. Smooth water limits (unless otherwise prescribed) 40 knots All 21 May 2004 Hire and drive personal 4. All Queensland waters 30 knots 27 May 2011 watercraft 5. Areas exempted from speed limit Note: this only applies if item 3 is the only valid speed limit for an area (a) the waters of Perserverance Dam, via Toowoomba Unlimited All 21 May 2004 (b) the waters of the Bjelke Peterson Dam at Murgon Unlimited All 21 May 2004 (c) the waters locally known as Sandy Hook Reach approximately Unlimited All 17 August 2010 between Branyan and Tyson Crossing on the Burnett River (d) the waters upstream of the Barrage on the Fitzroy River Unlimited All 21 May 2004 (e) the waters of Peter Faust Dam at Proserpine Unlimited All 21 May 2004 (f) the waters of Ross Dam at Townsville Unlimited All 9 October 2013 (g) the waters of Tinaroo Dam in the Atherton Tableland (unless Unlimited All 21 May 2004 otherwise prescribed) (h) the waters of Trinity Inlet in front of the Esplanade at Cairns Unlimited All 21 May 2004 (i) the waters of Marian Weir Unlimited All 21 May 2004 (j) the waters of Plantation Creek known as Hutchings Lagoon Unlimited All 21 May 2004 (k) the waters in Kinchant Dam at Mackay Unlimited All 21 May 2004 (l) the waters of Lake Maraboon at Emerald Unlimited All 6 May 2005 (m) the waters of Bundoora Dam, Middlemount 6 knots All 20 May 2016 6. -

Matters of NATIONAL Environmental Significance
AIRPOrt AND SURROUNds MATTERS OF NATIONAL EnVIROnmENTAL E2 SIGniFICANCE CONTENTS 1.6 Mitigation measures ......................................................433 1.7 Impact assessment summary ....................................435 Introduction to this Chapter ......................................................409 1.8 Conclusion .........................................................................435 SECTION 1: TERRESTRIAL FLORA SECTION 2: TERRESTRIAL FAUNA 1.1 Methodology and assumptions .................................. 410 2.1 Introduction .......................................................................436 1.1.1 Information and data collection ..........................410 2.2 Nomenclature and terminology ..................................436 1.1.1.1 Desktop studies .........................................410 1.1.1.2 Field surveys ................................................410 2.3 Methodology and assumptions ..................................436 1.1.1.3 Main survey ..................................................410 2.3.1 Desktop assessment and background sources ...............................................436 1.1.1.4 Supplementary spring surveys ...........413 2.3.2 Field survey overview ..............................................439 1.1.1.5 Vegetation mapping .................................413 2.3.3 Baseline terrestrial vertebrate survey ...............439 1.1.1.6 Assumptions and technical limitations ..................................413 2.3.3.1 Site selection (stratification) .................439 -

Predicting and Regulating Boat-Generated Waves Within Rivers and Sheltered Waterways
Predicting And Regulating Boat-generated Waves Within Rivers And Sheltered Waterways Associate Professor Gregor Macfarlane Australian Maritime College, University of Tasmania SYDNEY, AUSTRALIA | 14 -18 October 2018 MANAGED BY • All marine vessels generate waves. • Vessels now go faster, further and more often. • New issues for other users and the environment: • bank erosion; • damage to moored vessels, jetties and other marine structures; • endanger people working or enjoying activities in small craft or close to the shore. SYDNEY, AUSTRALIA | 14 -18 October 2018 MANAGED BY SYDNEY, AUSTRALIA | 14 -18 October 2018 MANAGED BY Gordon River, south-west Tasmania SYDNEY, AUSTRALIA | 14 -18 October 2018 MANAGED BY SYDNEY, AUSTRALIA | 14 -18 October 2018 MANAGED BY SYDNEY, AUSTRALIA | 14 -18 October 2018 MANAGED BY SYDNEY, AUSTRALIA | 14 -18 October 2018 MANAGED BY Test Sites Boat / Ship Wave Wake Test Sites : • Gordon River, Tasmania • Strahan Harbour, Tasmania • Tamar River, Tasmania • Derwent River, Tasmania • Sydney Harbour (5 locations), Sydney, New South Wales • Parramatta River, Sydney, New South Wales • Hunter River, Newcastle, New South Wales • Brisbane River, Bulimba, Brisbane, Queensland • Brisbane River, upstream of Bremer River, Queensland • Noosa River, Noosa, Brisbane, Queensland • Maroochy River, Maroochydore, Queensland • Swan River, Perth, Western Australia • Canning River, Perth, Western Australia • Daly River, Malak Malak region, Northern Territory • Willamette River, Oregon, United States of America SYDNEY, AUSTRALIA | 14 -18 October 2018 MANAGED BY Need to take action… • regulate vessel operations (vessel speed and/or route); • optimise the vessel design; or, • implement remedial measures on shore. SYDNEY, AUSTRALIA | 14 -18 October 2018 MANAGED BY Need to take action…and planning in the early stages • regulate vessel operations (vessel speed and/or route); • optimise the vessel design; or, • implement remedial measures on shore. -

Baddiley Peter Second Statement Annex PB2-816.Pdf
In the matter of the Commissions of Inquiry Act 1950 Commissions of Inquiry Order (No.1) 2011 Queensland Floods Commission of Inquiry Second Witness Statement of Peter Baddiley Annexure “PB2-8(16)” PB2-8(16) 1 PB2-8(16) 2 PB2-8 (16) FLDWARN Coastal Rs Maryborough south 1 December 2010 to 31 January 2011 TO::BOM612+BOM613+BOM614+BOM615+BOM617+BOM618 IDQ20780 Australian Government Bureau of Meteorology Queensland FLOOD WARNING FOR COASTAL STREAMS AND ADJACENT INLAND CATCHMENTS FROM MARYBOROUGH TO THE NSW BORDER Issued at 6:46 PM on Saturday the 11th of December 2010 by the Bureau of Meteorology, Brisbane. Heavy rainfall during Saturday has resulted in fast level rises in coastal catchments and adjacent inland catchments. The heaviest rainfall to 6pm Saturday has been in the Pine Rivers area and coastal areas from Brisbane to the Gold Coast. Further rainfall is forecast overnight with fast rises and some minor flooding expected. Rainfall totals in the 9 hours to 6pm include: Wynnum 100mm, Mitchelton 76mm, Logan 65mm, Coomera 46mm , Brisbane 74mm and Beerwah 60m. ## Next Issue: The next warning will be issued by 8am Sunday. Latest River Heights: nil. Warnings and River Height Bulletins are available at http://www.bom.gov.au/qld/flood/ . Flood Warnings are also available on telephone 1300 659 219 at a low call cost of 27.5 cents, more from mobile, public and satellite phones. TO::BOM612+BOM613+BOM614+BOM615+BOM617+BOM618 IDQ20780 Australian Government Bureau of Meteorology Queensland FLOOD WARNING FOR COASTAL STREAMS AND ADJACENT INLAND CATCHMENTS FROM MARYBOROUGH TO BRISBANE Issued at 8:19 AM on Sunday the 12th of December 2010 by the Bureau of Meteorology, Brisbane. -

Landscape Legacies: Koala Habitat Change in Noosa Shire, South-East Queensland L.M
Landscape legacies: Koala habitat change in Noosa Shire, South-east Queensland L.M. Seabrook1, C. A. McAlpine1, S. R. Phinn1, J. Callaghan2, and D. Mitchell2 1School of Geography, Planning and Architecture and The Ecology Centre, The University of Queensland, Brisbane, Queensland 4072 2The Australian Koala Foundation G.P.O. Box 2659, Brisbane 4001 Corresponding Author: [email protected] Present day Australian landscapes are legacies of our colonial history, while future landscapes will be legacies of ecological processes and human impacts occurring today. This paper investigates the legacies of European settlement of Noosa Shire, South-east Queensland, with particular emphasis on the economic and political drivers and the resultant loss and fragmentation of Koala Phascolarctos cinereus habitat. Patterns of habitat loss between 1860 and 1970 were quantified at a coarse level from historical and land tenure records, while changes over the past 30 years were mapped at a finer spatial resolution from aerial photography and satellite imagery. Periods of high economic growth and to lesser extents depression are linked to increased vegetation clearing. Fifty per cent of P. cinereus habitat has been lost since 1860, with habitat class 2A (30-<50 per cent of preferred habitat trees) and 2C (10-<30 per cent of preferred habitat trees) suffering the highest proportion of loss. The period of greatest habitat loss was between 1890 and 1910, linked to the development of the dairy industry in the western half of Noosa Shire. A second significant phase of loss occurred since 1970, linked to the planting of exotic pine plantations, urbanisation and rural subdivision, with 35 per cent of remaining habitat being cleared, mainly in the southern part of the Shire.