VIENNA BIOCENTER 2008 IMP COVER 2008 INNENSEITEN CONTENTS Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
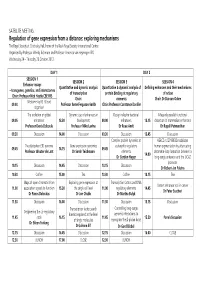
SM08 Programme
SATELLITE MEETING Regulation of gene expression from a distance: exploring mechanisms The Royal Society at Chicheley Hall, home of the Kavli Royal Society International Centre Organised by Professor Wendy Bickmore and Professor Veronica van Heyningen FRS Wednesday 24 – Thursday 25 October 2012 DAY 1 DAY 2 SESSION 1 SESSION 2 SESSION 3 SESSION 4 Enhancer assays Quantitative and dynamic analysis Quantitative & dynamic analysis of Defining enhancers and their mechanisms – transgenes, genetics, and interactomes of transcription protein binding at regulatory of action Chair: Professor Nick Hastie CBE FRS Chair: elements Chair: Dr Duncan Odom Welcome by RS & lead 09.00 Professor Anne Ferguson-Smith Chair: Professor Constance Bonifer organiser The evolution of global Dynamic use of enhancers in Design rules for bacterial Massively parallel functional 09.05 enhancers 13.30 development 09.00 enhancers 13.15 dissection of mammalian enhancers Professor Denis Duboule Professor Mike Levine Dr Roee Amit Dr Rupali Patwardhan 09.30 Discussion 14.00 Discussion 09.30 Discussion 13.45 Discussion Complex protein dynamics at HERC2 rs12913832 modulates The pluripotent 3D genome Gene expression genomics eukaryotic regulatory human pigmentation by attenuating 09.45 14.15 09.45 Professor Wouter de Laat Dr Sarah Teichmann elements chromatin-loop formation between a 14.00 Dr Gordon Hager long-range enhancer and the OCA2 promoter 10.15 Discussion 14.45 Discussion 10.15 Discussion Dr Robert-Jan Palstra 10.30 Coffee 15.00 Tea 10.30 Coffee 14.15 Tea Maps of open chromatin -

Gene Expression Studies: from Case-Control to Multiple-Population-Based Studies
From the Institute of Human Genetics, Helmholtz Zentrum Munchen,¨ Deutsches Forschungszentrum fur¨ Gesundheit und Umwelt (GmbH) Head: Prof. Dr. Thomas Meitinger Gene expression studies: From case-control to multiple-population-based studies Thesis Submitted for a Doctoral Degree in Natural Sciences at the Faculty of Medicine, Ludwig-Maximilians-Universitat¨ Munchen¨ Katharina Schramm Dachau, Germany 2016 With approval of the Faculty of Medicine Ludwig-Maximilians-Universit¨atM ¨unchen Supervisor/Examiner: Prof. Dr. Thomas Illig Co-Examiners: Prof. Dr. Roland Kappler Dean: Prof. Dr. med. dent. Reinhard Hickel Date of oral examination: 22.12.2016 II Dedicated to my family. III Abstract Recent technological developments allow genome-wide scans of gene expression levels. The reduction of costs and increasing parallelization of processing enable the quantification of 47,000 transcripts in up to twelve samples on a single microarray. Thereby the data collec- tion of large population-based studies was improved. During my PhD, I first developed a workflow for the statistical analyses of case-control stu- dies of up to 50 samples. With large population-based data sets generated I established a pipeline for quality control, data preprocessing and correction for confounders, which re- sulted in substantially improved data. In total, I processed more than 3,000 genome-wide expression profiles using the generated pipeline. With 993 whole blood samples from the population-based KORA (Cooperative Health Research in the Region of Augsburg) study we established one of the largest population-based resource. Using this data set we contributed to a number of transcriptome-wide association studies within national (MetaXpress) and international (CHARGE) consortia. -

EMBC Annual Report 2007
EMBO | EMBC annual report 2007 EUROPEAN MOLECULAR BIOLOGY ORGANIZATION | EUROPEAN MOLECULAR BIOLOGY CONFERENCE EMBO | EMBC table of contents introduction preface by Hermann Bujard, EMBO 4 preface by Tim Hunt and Christiane Nüsslein-Volhard, EMBO Council 6 preface by Marja Makarow and Isabella Beretta, EMBC 7 past & present timeline 10 brief history 11 EMBO | EMBC | EMBL aims 12 EMBO actions 2007 15 EMBC actions 2007 17 EMBO & EMBC programmes and activities fellowship programme 20 courses & workshops programme 21 young investigator programme 22 installation grants 23 science & society programme 24 electronic information programme 25 EMBO activities The EMBO Journal 28 EMBO reports 29 Molecular Systems Biology 30 journal subject categories 31 national science reviews 32 women in science 33 gold medal 34 award for communication in the life sciences 35 plenary lectures 36 communications 37 European Life Sciences Forum (ELSF) 38 ➔ 2 table of contents appendix EMBC delegates and advisers 42 EMBC scale of contributions 49 EMBO council members 2007 50 EMBO committee members & auditors 2007 51 EMBO council members 2008 52 EMBO committee members & auditors 2008 53 EMBO members elected in 2007 54 advisory editorial boards & senior editors 2007 64 long-term fellowship awards 2007 66 long-term fellowships: statistics 82 long-term fellowships 2007: geographical distribution 84 short-term fellowship awards 2007 86 short-term fellowships: statistics 104 short-term fellowships 2007: geographical distribution 106 young investigators 2007 108 installation -

Written Evidence Submitted by the MRC Human Genetics Unit at The
Written evidence submitted by the MRC Human Genetics Unit at the University of Edinburgh and the MRC Protein Phosphorylation and Ubiquitylation Unit at the University of Dundee (USC0001) We write in response to the UK Parliament Scottish Affairs Committee’s Call for Evidence in relation to Universities and Scotland. Our evidence pertains to “How Scottish university research fits in with UK university research”. We write as the directors of two UKRI Medical Research Council (MRC) Units based in Scottish Universities; the MRC Human Genetics Unit at the University of Edinburgh (https://www.ed.ac.uk/mrc-human-genetics-unit) and the MRC Protein Phosphorylation and Ubiquitylation Unit at the University of Dundee (https://www.ppu.mrc.ac.uk). As part of UKRI, the MRC invests 40% of its annual budget on an intramural portfolio of institutes, units and centres. MRC Units are long term investments that tackle important, often high risk, research questions that cannot be addressed adequately through short-term response-mode grant funding. MRC Units develop new methods and research facilities, foster interdisciplinarity, and spearhead training of the next generation of research leaders. Most MRC Units are embedded within universities (MRC University Units) and, because of the scale and sustainability of their research funding - reviewed and renewed every 5 years, they have a major impact on the research landscape of the universities that they are embedded in, including in Scotland. The scale and quality of research funded in MRC Units greatly enhances academic leadership within the host university as well as the Research Excellence Grant (REG) income that the host university receives from the Scottish Funding Council. -

Lectures and Seminars, Michaelmas Term 2014
WEDNESDay 8 octobEr 2014 • SUPPLEMENt (1) to No 5071 • VoL 145 Gazette Supplement Lectures and Seminars, Michaelmas term 2014 Romanes Lecture 26 Pharmacology, anatomical reuters Institute for the Study of Neuropharmacology and Drug Journalism Discovery Seminars Latin american centre Charles Simonyi Lecture 26 Physiology, anatomy and Genetics Foundation for Law, Justice and Society Population Health Maison Française Humanities 26 Psychiatry oxford Martin School Museum of Natural History TORCH | The Oxford Research Centre in Social Sciences 35 the Humanities Population ageing Classics anthropology and Museum Ethnography Ian ramsey centre English Language and Literature Archaeology Rhodes House English /History/History of Art/Theology/ Education McDonald centre for theology, Ethics and Public Life Music International Development (Queen History Elizabeth House) Colleges, Halls and Societies 47 History of Art Internet Institute Linguistics, Philology and Phonetics Law All Souls Medieval and Modern Languages Politics and International relations Green templeton Music Social Policy and Intervention Keble Oriental Studies Socio-legal Studies Kellogg Philosophy Sociology Mansfield Theology and Religion Nuffield Department for Continuing St Antony’s Education 40 Mathematical, Physical and St Catherine’s Life Sciences 32 Kellogg college centre for creative St Edmund Hall Chemistry Writing St Hilda’s Earth Sciences St John’s Institutes, Centres and Engineering Science Somerville Museums 41 e-Research Centre University college Materials ashmolean Museum -

Smutty Alchemy
University of Calgary PRISM: University of Calgary's Digital Repository Graduate Studies The Vault: Electronic Theses and Dissertations 2021-01-18 Smutty Alchemy Smith, Mallory E. Land Smith, M. E. L. (2021). Smutty Alchemy (Unpublished doctoral thesis). University of Calgary, Calgary, AB. http://hdl.handle.net/1880/113019 doctoral thesis University of Calgary graduate students retain copyright ownership and moral rights for their thesis. You may use this material in any way that is permitted by the Copyright Act or through licensing that has been assigned to the document. For uses that are not allowable under copyright legislation or licensing, you are required to seek permission. Downloaded from PRISM: https://prism.ucalgary.ca UNIVERSITY OF CALGARY Smutty Alchemy by Mallory E. Land Smith A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY GRADUATE PROGRAM IN ENGLISH CALGARY, ALBERTA JANUARY, 2021 © Mallory E. Land Smith 2021 MELS ii Abstract Sina Queyras, in the essay “Lyric Conceptualism: A Manifesto in Progress,” describes the Lyric Conceptualist as a poet capable of recognizing the effects of disparate movements and employing a variety of lyric, conceptual, and language poetry techniques to continue to innovate in poetry without dismissing the work of other schools of poetic thought. Queyras sees the lyric conceptualist as an artistic curator who collects, modifies, selects, synthesizes, and adapts, to create verse that is both conceptual and accessible, using relevant materials and techniques from the past and present. This dissertation responds to Queyras’s idea with a collection of original poems in the lyric conceptualist mode, supported by a critical exegesis of that work. -

Acknowledgment of Reviewers, 2015
Acknowledgment of Reviewers, 2015 The PNAS editors would like to thank all the individuals who dedicated their considerable time and expertise to the journal by serving as reviewers in 2015. Their generous contribution is deeply appreciated. A Peter B. Adler Colin J. Akerman Eric E. Allen James Ammerman Duur K. Aanen Ralph Adolphs Joshua M. Akey Heather C. Allen David M. Amodio Adam R. Abate Ruedi Aebersold Anna Akhmanova Jim Allen Valentin Amrhein John T. Abatzoglou Hugo Aerts Hajime Akimoto Karen N. Allen Esther Amstad Jonathan Abbatt Hagit P. Affek Akin Akinc Michael F. Allen Ronald Amundson Allison Abbott Arash Afraz Shizuo Akira Paul M. Allen Weihua An Jeffrey Abbott Theodor Agapie Ozan Akkus Rosalind J. Allen Zhiqiang An Larry F. Abbott David A. Agard Ivona Aksentijevich Morten Erik Allentoft Laura Diaz Anadon Nicholas L. Abbott Sapan Agarwal Serap Aksoy Stefano Allesina Ganesh Srinivasan Anand Chaouki T. Abdallah Joel W. Ager III Yousef Al-Abed David B. Allison Cort Anastasio Omar Abdel-Wahab Ingi Agnarsson Ashraf Al-Amoudi Steven D. Allison Lefteris Jason Ikuro Abe Anurag A. Agrawal Eric E. Alani Julian M. Allwood Anastasopoulos Stephen Tobias Abedon Ashutosh Agrawal Balbino Alarcón Eric J. Alm Hossain Anawar Moshe Abeles Rakesh Agrawal Qais Al-Awqati Benjamin A. Alman Elissar Andari Asa Abeliovich Jon Ågren Joseph Albanesi Ingvild Almas William R. L. Anderegg John Aber Alan Agresti Francis Albarede Steven C. Almo John M. Anderies Clara Abraham Jeremy J. Agresti Umberto Albarella Douglas Almond Mark L. Andermann John Abraham Jay J. Ague Silas D. Alben Uri Alon Bogi Andersen Daniel A. Abrams Fernan Agüero Frank Alber José M. -

Directory 2016/17 the Royal Society of Edinburgh
cover_cover2013 19/04/2016 16:52 Page 1 The Royal Society of Edinburgh T h e R o Directory 2016/17 y a l S o c i e t y o f E d i n b u r g h D i r e c t o r y 2 0 1 6 / 1 7 Printed in Great Britain by Henry Ling Limited, Dorchester, DT1 1HD ISSN 1476-4334 THE ROYAL SOCIETY OF EDINBURGH DIRECTORY 2016/2017 PUBLISHED BY THE RSE SCOTLAND FOUNDATION ISSN 1476-4334 The Royal Society of Edinburgh 22-26 George Street Edinburgh EH2 2PQ Telephone : 0131 240 5000 Fax : 0131 240 5024 email: [email protected] web: www.royalsoced.org.uk Scottish Charity No. SC 000470 Printed in Great Britain by Henry Ling Limited CONTENTS THE ORIGINS AND DEVELOPMENT OF THE ROYAL SOCIETY OF EDINBURGH .....................................................3 COUNCIL OF THE SOCIETY ..............................................................5 EXECUTIVE COMMITTEE ..................................................................6 THE RSE SCOTLAND FOUNDATION ..................................................7 THE RSE SCOTLAND SCIO ................................................................8 RSE STAFF ........................................................................................9 LAWS OF THE SOCIETY (revised October 2014) ..............................13 STANDING COMMITTEES OF COUNCIL ..........................................27 SECTIONAL COMMITTEES AND THE ELECTORAL PROCESS ............37 DEATHS REPORTED 26 March 2014 - 06 April 2016 .....................................................43 FELLOWS ELECTED March 2015 ...................................................................................45 -

EMBC Annual Report 2008
EMBO | EMBC annual report 2008 EUROPEAN MOLECULAR BIOLOGY ORGANIZATION | EUROPEAN MOLECULAR BIOLOGY CONFERENCE EMBO | EMBC table of contents introduction preface by Hermann Bujard, EMBO 5 preface by Tim Hunt, EMBO Council 8 preface by Peter Weisbeek & Krešimir Pavelić, EMBC 9 past & present timeline & brief history 12 EMBO | EMBC | EMBL aims 14 EMBO actions 2008 17 EMBC actions 2008 21 EMBO & EMBC programmes and activities fellowship programme 24 courses & workshops programme 25 young investigator programme 26 installation grants 27 science & society programme 28 EMBO activities The EMBO Journal 32 EMBO reports 33 Molecular Systems Biology 34 EMBO Molecular Medicine 35 journal subject categories 36 national science reviews 37 The EMBO Meeting 38 women in science 39 gold medal 40 award for communication in the life sciences 41 plenary lectures 42 information support & resources 43 public relations & communications 44 European Life Sciences Forum (ELSF) 45 ➔ 2 table of contents appendix EMBC delegates and advisers 48 EMBC scale of contributions 55 EMBO council members 2008 56 EMBO committee members & auditors 2008 57 EMBO council members 2009 58 EMBO committee members & auditors 2009 59 EMBO members elected in 2008 60 advisory editorial boards & senior editors 2008 72 long-term fellowship awards 2008 76 long-term fellowships: statistics 94 long-term fellowships 2008: geographical distribution 96 short-term fellowship awards 2008 98 short-term fellowships: statistics 116 short-term fellowships 2008: geographical distribution 118 young investigators -

EMBO Facts & Figures
excellence in life sciences young investigators|courses,workshops,conference series & symposia|installation grantees|long-term fellows|short-term fellows|policy, science & society|the EMBO Journal|EMBO reports|molecular systems biology|EMBO molecular medicine|global exchange|gold medal|the EMBO meeting|women in science| EMBO reports|molecular systems biology|EMBO molecular medicine|global exchange|gold medal|the EMBO meeting|women in science|young investigators|courses,workshops,conference series & symposia|installation grantees|long-term fellows|short-term fellows|policy, science & society|the EMBO Journal| global exchange|gold medal|the EMBO meeting|women in science|young investigators|long-term fellows|short-term fellows|policy, science & society|the EMBO Journal|courses,workshops,conference series & symposia|EMBO reports|molecular systems biology|EMBO molecular medicine|installation grantees| EMBO molecular medicine|installation grantees|long-term fellows|gold medal|molecular systems biology|short-term fellows|the EMBO meeting|womenReykjavik in science|young investigators|courses,workshops,conference series & symposia|global exchange|EMBO reports|policy, science & society|the EMBO Journal| gold medal|the EMBO meeting|women in science|young investigators|courses,workshops,conference series & symposia|global exchange|policy, science & society|the EMBO Journal|EMBO reports|molecular systems biology|EMBO molecular medicine|installation grantees|long-term fellows|short-term fellows| courses,workshops,conference series & symposia|global -

The Chronicle 2015-2016
St Hugh’s College Oxford A record of news, events and achievements within the St Hugh’s College community for the academic year 2015-16 CHRONICLE October 2015 - September 2016 ST HUGH’S COLLEGE CHRONICLE 2015-16 Editorial Helen Popescu (English, 2006), Publications Officer The writing of this Editorial could not come at a more appropriate time for me. In the ten years since I matriculated, I have been fortunate to remain involved with College throughout. Now, as I prepare to leave my role as Publications Officer, it is a great pleasure to reflect on how the College has evolved and grown in this time. I hope that this Chronicle evinces some of the great strengths of the past academic year, and gives reason to trust that these can be matched and surpassed in the year to come. Many of the reports in the Chronicle, and particularly those of the JCR and MCR, celebrate the diversity of human experience generated by bringing together gifted students from an enormous variety of backgrounds. This has always been, and must remain, the core of College’s purpose, especially when the uncertainty following the decision for the UK to leave the European Union has been so pervasive. This year’s ‘Articles by Alumni’ focus on unusual paths taken after leaving College. If I could share any advice with my first year undergraduate self, it would be that choosing an uncommon career trajectory, although daunting, is also incredibly gratifying. It was such a delight this year to receive many reports of our alumni’s achievements in a variety of different fields – thank you to all those of you who shared your news. -

A Century of Genetics - Programme
A Century of Genetics - Programme Wednesday 13 November 09:00-09:30 Registration 09:30-09:40 Introduction to the conference Laurence Hurst, Genetics Society Eleanor Riley, Roslin Institute Session 1: Genome stability and instability Chair: David Finnegan, co-chair Sveta Markovets 09:40-09:45 Introduction to the session David Finnegan 09:45-10:15 Steve Jackson (University of Cambridge) 10:15-10:40 Severine Chambeyron (Institute of Human Genetics, Montpelier) 10:40-11:15 Refreshment break 11:15-11:45 Kim Nasmyth (University of Oxford) 11:45-12:10 Kirsten Bomblies (John Innes Centre) 12:10-12:25 Speaker selected from abstracts 12:25-12:45 Poster pitches x5 12:45-14:00 Lunch break Poster session - even numbers Session 2: Genetics and selection of quantitative traits Chair: Josephine Pemberton, co-chair Smaragda Tsairidou Sponsor: Aviagen 14:00-14:05 Introduction to the session Josephine Pemberton 14:05-14:35 Trudy MacKay (NCSU/Clemson) 14:35-14:50 Selected speaker 14:50-15:05 Selected speaker 15:05-15:30 Felicity Jones (Tuebingen) 15:30-16:00 Refreshment break 16:00-16:30 John Hickey (Roslin Institute) 16:30-16:55 Balfour Lecture: Susan Johnston (University of Edinburgh) Introduced by Laurence Hurst 16:55-17:10 Selected speaker 17:10-17:25 Selected speaker 17:25-17:50 Greg Kudla (University of Edinburgh) 17:50-18:30 Mendel Medal: Professor Bill Hill Introduced by Laurence Hurst Appreciation of Bill’s work by Trudy Mackay 18:30-20:00 Drinks reception with posters on display Sponsored by Genus PLC 19:30-21:00 Public Lecture Farm, Field and