The University of Chicago Interrogating the 3D Structure of Primate Genomes a Dissertation Submitted to the Faculty of the Divis
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
SM08 Programme
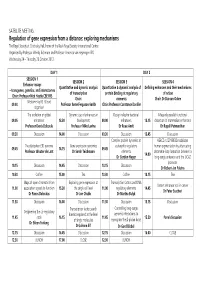
SATELLITE MEETING Regulation of gene expression from a distance: exploring mechanisms The Royal Society at Chicheley Hall, home of the Kavli Royal Society International Centre Organised by Professor Wendy Bickmore and Professor Veronica van Heyningen FRS Wednesday 24 – Thursday 25 October 2012 DAY 1 DAY 2 SESSION 1 SESSION 2 SESSION 3 SESSION 4 Enhancer assays Quantitative and dynamic analysis Quantitative & dynamic analysis of Defining enhancers and their mechanisms – transgenes, genetics, and interactomes of transcription protein binding at regulatory of action Chair: Professor Nick Hastie CBE FRS Chair: elements Chair: Dr Duncan Odom Welcome by RS & lead 09.00 Professor Anne Ferguson-Smith Chair: Professor Constance Bonifer organiser The evolution of global Dynamic use of enhancers in Design rules for bacterial Massively parallel functional 09.05 enhancers 13.30 development 09.00 enhancers 13.15 dissection of mammalian enhancers Professor Denis Duboule Professor Mike Levine Dr Roee Amit Dr Rupali Patwardhan 09.30 Discussion 14.00 Discussion 09.30 Discussion 13.45 Discussion Complex protein dynamics at HERC2 rs12913832 modulates The pluripotent 3D genome Gene expression genomics eukaryotic regulatory human pigmentation by attenuating 09.45 14.15 09.45 Professor Wouter de Laat Dr Sarah Teichmann elements chromatin-loop formation between a 14.00 Dr Gordon Hager long-range enhancer and the OCA2 promoter 10.15 Discussion 14.45 Discussion 10.15 Discussion Dr Robert-Jan Palstra 10.30 Coffee 15.00 Tea 10.30 Coffee 14.15 Tea Maps of open chromatin -

Ismb/Eccb 2015
Research Collection Journal Article ISMB/ECCB 2015 Author(s): Moreau, Yves; Beerenwinkel, Niko Publication Date: 2015 Permanent Link: https://doi.org/10.3929/ethz-b-000102416 Originally published in: Bioinformatics 31(12), http://doi.org/10.1093/bioinformatics/btv303 Rights / License: Creative Commons Attribution-NonCommercial 4.0 International This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Bioinformatics, 31, 2015, i1–i2 doi: 10.1093/bioinformatics/btv303 ISMB/ECCB 2015 Editorial ISMB/ECCB 2015 This special issue of Bioinformatics serves as the proceedings of the 175 external reviewers recruited as sub-reviewers by program com- joint 23rd annual meeting of Intelligent Systems for Molecular mittee members. Table 1 provides a summary of the areas, area Biology (ISMB) and 14th European Conference on Computational chairs and a review summary by area. The conference used a two- Biology (ECCB), which took place in Dublin, Ireland, July 10–14, tier review system—a continuation and refinement of a process that 2015 (http://www.iscb.org/ismbeccb2015). ISMB/ECCB 2015, the begun with ISMB/ECCB 2013 in an effort to better ensure thorough official conference of the International Society for Computational and fair reviewing. Under the revised process, each of the 241 sub- Biology (ISCB, http://www.iscb.org/), was accompanied by nine missions was first reviewed by at least three expert referees, with a Special Interest Group meetings of 1 or 2 days each, and two satel- subset receiving between four and six reviews, as needed. lite meetings. -

Gene Expression Studies: from Case-Control to Multiple-Population-Based Studies
From the Institute of Human Genetics, Helmholtz Zentrum Munchen,¨ Deutsches Forschungszentrum fur¨ Gesundheit und Umwelt (GmbH) Head: Prof. Dr. Thomas Meitinger Gene expression studies: From case-control to multiple-population-based studies Thesis Submitted for a Doctoral Degree in Natural Sciences at the Faculty of Medicine, Ludwig-Maximilians-Universitat¨ Munchen¨ Katharina Schramm Dachau, Germany 2016 With approval of the Faculty of Medicine Ludwig-Maximilians-Universit¨atM ¨unchen Supervisor/Examiner: Prof. Dr. Thomas Illig Co-Examiners: Prof. Dr. Roland Kappler Dean: Prof. Dr. med. dent. Reinhard Hickel Date of oral examination: 22.12.2016 II Dedicated to my family. III Abstract Recent technological developments allow genome-wide scans of gene expression levels. The reduction of costs and increasing parallelization of processing enable the quantification of 47,000 transcripts in up to twelve samples on a single microarray. Thereby the data collec- tion of large population-based studies was improved. During my PhD, I first developed a workflow for the statistical analyses of case-control stu- dies of up to 50 samples. With large population-based data sets generated I established a pipeline for quality control, data preprocessing and correction for confounders, which re- sulted in substantially improved data. In total, I processed more than 3,000 genome-wide expression profiles using the generated pipeline. With 993 whole blood samples from the population-based KORA (Cooperative Health Research in the Region of Augsburg) study we established one of the largest population-based resource. Using this data set we contributed to a number of transcriptome-wide association studies within national (MetaXpress) and international (CHARGE) consortia. -
Published Version
PUBLISHED VERSION Katie L Ayers, Nadia M Davidson, Diana Demiyah, Kelly N Roeszler, Frank Grützner, Andrew H Sinclair, Alicia Oshlack and Craig A Smith RNA sequencing reveals sexually dimorphic gene expression before gonadal differentiation in chicken and allows comprehensive annotation of the W-chromosome Genome Biology (Print): biology for the post-genomic era, 2013; 14(3):R26 © 2013 Ayers et al.; licensee Springer. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Originally published at: http://doi.org/10.1186/gb-2013-14-3-r26 PERMISSIONS http://creativecommons.org/licenses/by/2.0/ http://hdl.handle.net/2440/82599 RNA sequencing reveals sexually dimorphic gene expression before gonadal differentiation in chicken and allows comprehensive annotation of the W-chromosome Ayers et al. Ayers et al. Genome Biology 2013, 14:R26 http://genomebiology.com/2013/14/3/R26 (25 March 2013) Ayers et al. Genome Biology 2013, 14:R26 http://genomebiology.com/2013/14/3/R26 RESEARCH Open Access RNA sequencing reveals sexually dimorphic gene expression before gonadal differentiation in chicken and allows comprehensive annotation of the W-chromosome Katie L Ayers1,2,3†, Nadia M Davidson1†, Diana Demiyah4, Kelly N Roeszler1, Frank Grützner5, Andrew H Sinclair1,2,6, Alicia Oshlack1* and Craig A Smith1,2,6* Abstract Background: Birds have a ZZ male: ZW female sex chromosome system and while the Z-linked DMRT1 gene is necessary for testis development, the exact mechanism of sex determination in birds remains unsolved. -

The for Report 07-08
THE CENTER FOR INTEGRATIVE GENOMICS REPORT 07-08 www.unil.ch/cig Table of Contents INTRODUCTION 2 The CIG at a glance 2 The CIG Scientific Advisory Committee 3 Message from the Director 4 RESEARCH 6 Richard Benton Chemosensory perception in Drosophila: from genes to behaviour 8 Béatrice Desvergne Networking activity of PPARs during development and in adult metabolic homeostasis 10 Christian Fankhauser The effects of light on plant growth and development 12 Paul Franken Genetics and energetics of sleep homeostasis and circadian rhythms 14 Nouria Hernandez Mechanisms of basal and regulated RNA polymerase II and III transcription of ncRNA in mammalian cells 16 Winship Herr Regulation of cell proliferation 18 Henrik Kaessmann Mammalian evolutionary genomics 20 Sophie Martin Molecular mechanisms of cell polarization 22 Liliane Michalik Transcriptional control of tissue repair and angiogenesis 24 Alexandre Reymond Genome structure and expression 26 Andrzej Stasiak Functional transitions of DNA structure 28 Mehdi Tafti Genetics of sleep and the sleep EEG 30 Bernard Thorens Molecular and physiological analysis of energy homeostasis in health and disease 32 Walter Wahli The multifaceted roles of PPARs 34 Other groups at the Génopode 37 CORE FACILITIES 40 Lausanne DNA Array Facility (DAFL) 42 Protein Analysis Facility (PAF) 44 Core facilities associated with the CIG 46 EDUCATION 48 Courses and lectures given by CIG members 50 Doing a PhD at the CIG 52 Seminars and symposia 54 The CIG annual retreat 62 The CIG and the public 63 Artist in residence at the CIG 63 PEOPLE 64 1 Introduction The Center for IntegratiVE Genomics (CIG) at A glance The Center for Integrative Genomics (CIG) is the newest depart- ment of the Faculty of Biology and Medicine of the University of Lausanne (UNIL). -

Ontology-Based Methods for Analyzing Life Science Data
Habilitation a` Diriger des Recherches pr´esent´ee par Olivier Dameron Ontology-based methods for analyzing life science data Soutenue publiquement le 11 janvier 2016 devant le jury compos´ede Anita Burgun Professeur, Universit´eRen´eDescartes Paris Examinatrice Marie-Dominique Devignes Charg´eede recherches CNRS, LORIA Nancy Examinatrice Michel Dumontier Associate professor, Stanford University USA Rapporteur Christine Froidevaux Professeur, Universit´eParis Sud Rapporteure Fabien Gandon Directeur de recherches, Inria Sophia-Antipolis Rapporteur Anne Siegel Directrice de recherches CNRS, IRISA Rennes Examinatrice Alexandre Termier Professeur, Universit´ede Rennes 1 Examinateur 2 Contents 1 Introduction 9 1.1 Context ......................................... 10 1.2 Challenges . 11 1.3 Summary of the contributions . 14 1.4 Organization of the manuscript . 18 2 Reasoning based on hierarchies 21 2.1 Principle......................................... 21 2.1.1 RDF for describing data . 21 2.1.2 RDFS for describing types . 24 2.1.3 RDFS entailments . 26 2.1.4 Typical uses of RDFS entailments in life science . 26 2.1.5 Synthesis . 30 2.2 Case study: integrating diseases and pathways . 31 2.2.1 Context . 31 2.2.2 Objective . 32 2.2.3 Linking pathways and diseases using GO, KO and SNOMED-CT . 32 2.2.4 Querying associated diseases and pathways . 33 2.3 Methodology: Web services composition . 39 2.3.1 Context . 39 2.3.2 Objective . 40 2.3.3 Semantic compatibility of services parameters . 40 2.3.4 Algorithm for pairing services parameters . 40 2.4 Application: ontology-based query expansion with GO2PUB . 43 2.4.1 Context . 43 2.4.2 Objective . -

EMBC Annual Report 2007
EMBO | EMBC annual report 2007 EUROPEAN MOLECULAR BIOLOGY ORGANIZATION | EUROPEAN MOLECULAR BIOLOGY CONFERENCE EMBO | EMBC table of contents introduction preface by Hermann Bujard, EMBO 4 preface by Tim Hunt and Christiane Nüsslein-Volhard, EMBO Council 6 preface by Marja Makarow and Isabella Beretta, EMBC 7 past & present timeline 10 brief history 11 EMBO | EMBC | EMBL aims 12 EMBO actions 2007 15 EMBC actions 2007 17 EMBO & EMBC programmes and activities fellowship programme 20 courses & workshops programme 21 young investigator programme 22 installation grants 23 science & society programme 24 electronic information programme 25 EMBO activities The EMBO Journal 28 EMBO reports 29 Molecular Systems Biology 30 journal subject categories 31 national science reviews 32 women in science 33 gold medal 34 award for communication in the life sciences 35 plenary lectures 36 communications 37 European Life Sciences Forum (ELSF) 38 ➔ 2 table of contents appendix EMBC delegates and advisers 42 EMBC scale of contributions 49 EMBO council members 2007 50 EMBO committee members & auditors 2007 51 EMBO council members 2008 52 EMBO committee members & auditors 2008 53 EMBO members elected in 2007 54 advisory editorial boards & senior editors 2007 64 long-term fellowship awards 2007 66 long-term fellowships: statistics 82 long-term fellowships 2007: geographical distribution 84 short-term fellowship awards 2007 86 short-term fellowships: statistics 104 short-term fellowships 2007: geographical distribution 106 young investigators 2007 108 installation -

PREDICTD: Parallel Epigenomics Data Imputation with Cloud-Based Tensor Decomposition
bioRxiv preprint doi: https://doi.org/10.1101/123927; this version posted April 4, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. PREDICTD: PaRallel Epigenomics Data Imputation with Cloud-based Tensor Decomposition Timothy J. Durham Maxwell W. Libbrecht Department of Genome Sciences Department of Genome Sciences University of Washington University of Washington J. Jeffry Howbert Jeff Bilmes Department of Genome Sciences Department of Electrical Engineering University of Washington University of Washington William Stafford Noble Department of Genome Sciences Department of Computer Science and Engineering University of Washington April 4, 2017 Abstract The Encyclopedia of DNA Elements (ENCODE) and the Roadmap Epigenomics Project have produced thousands of data sets mapping the epigenome in hundreds of cell types. How- ever, the number of cell types remains too great to comprehensively map given current time and financial constraints. We present a method, PaRallel Epigenomics Data Imputation with Cloud-based Tensor Decomposition (PREDICTD), to address this issue by computationally im- puting missing experiments in collections of epigenomics experiments. PREDICTD leverages an intuitive and natural model called \tensor decomposition" to impute many experiments si- multaneously. Compared with the current state-of-the-art method, ChromImpute, PREDICTD produces lower overall mean squared error, and combining methods yields further improvement. We show that PREDICTD data can be used to investigate enhancer biology at non-coding human accelerated regions. PREDICTD provides reference imputed data sets and open-source software for investigating new cell types, and demonstrates the utility of tensor decomposition and cloud computing, two technologies increasingly applicable in bioinformatics. -

Computational Biology and Bioinformatics
Vol. 30 ISMB 2014, pages i1–i2 BIOINFORMATICS EDITORIAL doi:10.1093/bioinformatics/btu304 Editorial This special issue of Bioinformatics serves as the proceedings of The conference used a two-tier review system, a continuation the 22nd annual meeting of Intelligent Systems for Molecular and refinement of a process begun with ISMB 2013 in an effort Biology (ISMB), which took place in Boston, MA, July 11–15, to better ensure thorough and fair reviewing. Under the revised 2014 (http://www.iscb.org/ismbeccb2014). The official confer- process, each of the 191 submissions was first reviewed by at least ence of the International Society for Computational Biology three expert referees, with a subset receiving between four and (http://www.iscb.org/), ISMB, was accompanied by 12 Special eight reviews, as needed. These formal reviews were frequently Interest Group meetings of one or two days each, two satellite supplemented by online discussion among reviewers and Area meetings, a High School Teachers Workshop and two half-day Chairs to resolve points of dispute and reach a consensus on tutorials. Since its inception, ISMB has grown to be the largest each paper. Among the 191 submissions, 29 were conditionally international conference in computational biology and bioinfor- accepted for publication directly from the first round review Downloaded from matics. It is expected to be the premiere forum in the field for based on an assessment of the reviewers that the paper was presenting new research results, disseminating methods and tech- clearly above par for the conference. A subset of 16 papers niques and facilitating discussions among leading researchers, were viewed as potentially in the top tier but raised significant practitioners and students in the field. -

2016 Winter School Program
2016 Winter School in Mathematical & Computational Biology 4-8 July 2016 Auditorium Queensland Bioscience Precinct The University of Queensland Brisbane, Australia Program Hosted by: IMB 2016 Winter School in Mathematical and Computational Biology 4-‐8 July 2016 http://bioinformatics.org.au/ws16 Queensland Bioscience Precinct (Building #80) The University of Queensland Brisbane, Australia MONDAY 4 JULY 2016 08:00 Registration desk open NEXT GENERATION SEQUENCING & BIOINFORMATICS 09:00 – 09:05 Welcome and introduction Dr Nicholas Hamilton Research Computing Centre and Institute for Molecular Bioscience The University of Queensland 09:05 – 09:45 Next-‐generation sequencing overview (Game of Thrones Edition) Dr Ken McGrath Australian Genome Research Facility Ltd, Brisbane 09:45 – 10:30 NGS mapping, errors and quality control Dr Felicity Newell Queensland University of Technology, Brisbane 10:30 – 11:00 Morning Tea 11:00 – 11:45 Mutation detection in -‐ whole genome sequencing Dr Ann-‐Marie Patch QIMR Berghofer Medical Research Institute, Brisbane 11:45 – 12:30 De novo genome assembly A/Professor Torsten Seemann Victorian Life Sciences Computation , Initiative The University of Melbourne 12:30 – 13:30 Lunch 13:30 – 14:30 Long-‐read sequencing: an overview of technologies and applications Dr Mathieu Bourgey Montréal Node, McGill University and Genome Québec Innovation Centre, Canada 14:30 – 15:15 Genomics resources -‐ feeding your inner bioinformatician A/Professor Mik Black University of Otago, Dunedin, New Zealand 15:15 – 15:45 Afternoon -

EYAL AKIVA, Phd
Eyal Akiva CV, Nov. 2017 EYAL AKIVA, PhD Department of Bioengineering and Therapeutic Sciences Phone +1-650-504-9008 University of California at San Francisco Email [email protected] 1700 4th street, San Francisco, Web www.babbittlab.ucsf.edu/eakiva CA, USA EDUCATION 2012-2017 Post-doctoral fellowship at UCSF, Dept. Of Bioengineering and Therapeutic Sciences. Host: Prof. Patricia Babbitt. 2010-2012 Post-doctoral fellowship at UCSF, Dept. Of Bioengineering and Therapeutic Sciences. Host: Prof. Tanja Kortemme. 2004-2010 PhD at The Hebrew University of Jerusalem (Israel), bioinformatics. Host: Prof. Hanah Margalit. “Various Aspects of Modularity in Protein-Protein Interaction". 2001-2004 MSc at The Hebrew University of Jerusalem (Israel), bioinformatics and human genetics. Host: Prof. Muli Ben-Sasson. “Exploiting the Exploiters: Identification of Virus-Host Pep- tide Mimicry as a Source for Modules of Functional Significance”. MAGNA CUM LAUDE. 1997-2000 BSc at Bar-Ilan University (Israel), biology (major) and computer science (minor). Final project advisor: Prof. Ramit Mehr “Modeling the Evolution of the Immune System: a Sim- ulation of the Evolution of Genes that Encode the Variable Regions of Immunoglobulins”. MAGNA CUM LAUDE. 1996-1997 First year of "Industrial Engineering and Management" studies, Tel-Aviv University, Israel. OTHER WORK EXPERIENCE 2000-01 ‘Do-coop technologies’: Team leader and chemistry/microbiology researcher; development of biological applications and manufacture of proprietary nanoparticles (Or Yehuda, Israel and Tel-Aviv University (Prof. Eshel Ben-Jacob’s lab at the school of physics)). FUNDING, HONORS AND AWARDS 2017 Grant: Co-PI, “Utilizing metagenomic sequences for enzyme function prediction”, Joint Genome Institute (US Department of Energy) (http://jgi.doe.gov/doe-user-facilities-ficus- join-forces-to-tackle-biology-big-data/). -

RNA-Seq Are Likely The
bioRxiv preprint doi: https://doi.org/10.1101/110148; this version posted February 20, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Evolinc: a comparative transcriptomics and genomics pipeline for quickly identifying sequence conserved lincRNAs for functional analysis. Andrew D. L. Nelson*,1,†, Upendra K. Devisetty*,2, Kyle Palos1, Asher K. Haug-Baltzell3, Eric Lyons2,3, and Mark A. Beilstein1,† Authors: 1School of Plant Sciences, University of Arizona, Tucson, Arizona, 85721, 2 CyVerse, Bio5, University of Arizona, Tucson, Arizona, 85721, 3Genetics Graduate Interdisciplinary Group, University of Arizona, Tucson, Arizona, 85721 * These authors contributed equally to this manuscript. † Corresponding Authors Corresponding Authors: Mark Beilstein, 1140 E. South Campus Drive, 303 Forbes Building, Tucson, Arizona, 85721-0036, 520-626-1562, [email protected] Andrew Nelson, 1140 E. South Campus Drive, 303 Forbes Building, Tucson, Arizona, 85721-0036, 520-626-1563, [email protected] bioRxiv preprint doi: https://doi.org/10.1101/110148; this version posted February 20, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract Long intergenic non-coding RNAs (lincRNAs) are an abundant and functionally diverse class of eukaryotic transcripts. Reported lincRNA repertoires in mammals vary, but are commonly in the thousands to tens of thousands of transcripts, covering ~90% of the genome.