Mammalian Taxonomy and Community Ecology of a Neotropical Mountain Range
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rodentia: Cricetidae: Sigmodontinae) in São Paulo State, Southeastern Brazil: a Locally Extinct Species?
Volume 55(4):69‑80, 2015 THE PRESENCE OF WILFREDOMYS OENAX (RODENTIA: CRICETIDAE: SIGMODONTINAE) IN SÃO PAULO STATE, SOUTHEASTERN BRAZIL: A LOCALLY EXTINCT SPECIES? MARCUS VINÍCIUS BRANDÃO¹ ABSTRACT The Rufous-nosed Mouse Wilfredomys oenax is a rare Sigmodontinae rodent known from scarce records from northern Uruguay and south and southeastern Brazil. This species is under- represented in scientific collections and is currently classified as threathened, being considered extinct at Curitiba, Paraná, the only confirmed locality of the species at southeastern Brazil. Although specimens from São Paulo were already reported, the presence of this species in this state seems to have passed unnoticed in recent literature. Through detailed morphological ana- lyzes of specimens cited in literature, the present work confirms and discusses the presence of this species in São Paulo state from a specimen collected more than 70 years ago. Recently, by the use of modern sampling methods, other rare Sigmodontinae rodents, such as Abrawayomys ruschii, Phaenomys ferrugineous and Rhagomys rufescens, have been recorded to São Paulo state. However, no specimen of Wilfredomys oenax has been recently reported indicating that this species might be locally extinct. The record mentioned here adds another species to the state of São Paulo mammal diversity and reinforces the urgency of studying Wilfredomys oenax. Key-Words: Atlantic Forest; Scientific collection; Threatened species. INTRODUCTION São Paulo is one the most studied states in Brazil regarding to fauna. Mammal lists from this state have Mammal species lists based on voucher-speci- been elaborated since the late XIX century (Von Iher- mens and literature records are essential for offering ing, 1894; Vieira, 1944a, b, 1946, 1950, 1953; Vivo, groundwork to understand a species distribution and 1998). -

Proquest Dissertations
The Neotropical rodent genus Rhipidom ys (Cricetidae: Sigmodontinae) - a taxonomic revision Christopher James Tribe Thesis submitted for the degree of Doctor of Philosophy University College London 1996 ProQuest Number: 10106759 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest. ProQuest 10106759 Published by ProQuest LLC(2016). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. Microform Edition © ProQuest LLC. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 ABSTRACT South American climbing mice and rats, Rhipidomys, occur in forests, plantations and rural dwellings throughout tropical South America. The genus belongs to the thomasomyine group, an informal assemblage of plesiomorphous Sigmodontinae. Over 1700 museum specimens were examined, with the aim of providing a coherent taxonomic framework for future work. A shortage of discrete and consistent characters prevented the use of strict cladistic methodology; instead, morphological assessments were supported by multivariate (especially principal components) analyses. The morphometric data were first assessed for measurement error, ontogenetic variation and sexual dimorphism; measurements with most variation from these sources were excluded from subsequent analyses. The genus is characterized by a combination of reddish-brown colour, long tufted tail, broad feet with long toes, long vibrissae and large eyes; the skull has a small zygomatic notch, squared or ridged supraorbital edges, large oval braincase and short palate. -

Chec List ISSN 1809-127X (Available at Journal of Species Lists and Distribution
Check List 8(3): 557-559, 2012 © 2012 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution N Rhagomys rufescens Sigmodontinae) Thomas, 1886 ISTRIBUTIO New distribution reports of (Rodentia: D * and Maria Rita Silvério Pires RAPHIC G Caryne Aparecida de Carvalho Braga EO G N Universidade Federal de Ouro Preto, Departamento de Evolução, Biodiversidade e Meio Ambiente, Laboratório de Zoologia dos Vertebrados, O [email protected] Campus Morro do Cruzeiro. CEP 35400-000. Ouro Preto, MG, Brasil. * Corresponding author. E-mail: OTES N Abstract: Rhagomys rufescens is a rare, arboreal sigmodontine rodent endemic to the Brazilian Atlantic Forest biome. This species is known from eight localities in Brazil. Here we present a new report based on four individuals of this species registered in Serra do Ouro Branco, municipality of Ouro Branco (Minas Gerais, Brazil). One juvenile male, one adult male ofand the two last juvenile registered females location. were captured in pitfall traps during the rainy season, in a study of small mammal ecology. This is the first record for the Espinhaço Mountain range and the northernmost report for the species in this state, 85 km northeast Rhagomys rufescens (Thomas, 1886) is an enigmatic level as “incertae sedis” (Reig 1980; 1984; McKenna and Forest domain, at the south of the Espinhaço mountain arboreal sigmodontine rodent classified at the suprageneric range. Our report occurred in an area of vegetation characterized as semi-deciduous mountain forest. A Bell 1997; Smith and Patton 1999; Musser and Carleton portion of the sampled locality is now inside a conservation 2005). -
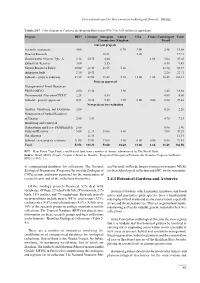
CBD First National Report
First national report for the Convention on Biological Diversity - BRAZIL Tabela 2-37. Pilot Program to Conserve the Brazilian Rain Forest PPG-7 (In US$ million or equivalent). Projects RFT* Germany European United USA France Counterpart Total Commission Kingdom Brazil Current projects Scientific institutions 9.00 0.70 3.00 2.98 15.68 Directed Research 10.91 9.00 19.91 Demonstrative Projects Type A 3.18 20.75 4.44 1.68 3.00 33.05 Extractivist Reserves 3.00 5.55 0.90 9.45 Natural Resources Policy 20.00 28.48 18.55 5.00 11.40 83.43 Indigenous lands 2.10 18.41 2.20 22.71 Subtotal – projects underway 37.28 67.64 39.45 5.70 12.00 1.68 20.48 184.23 Projects approved Management of Forest Resources- PROMANEJO 2.00 13.54 1.90 1.40 18.84 Environmental Education-CEDUC 2.25 5.55 0.80 8.60 Subtotal - projects approved 4.25 13.54 5.55 1.90 0.00 0.00 2.20 27.44 New projects for evaluation Analysis, Monitoring and Evaluation 2.00 0.20 2.20 Management of Natural Resources of Várzeas 2.00 4.54 0.70 7.24 Monitoring and Control of Deforestation and Fires- PRODESQUE 2.00 0.90 2.90 Parks and Reserves 5.00 21.15 13.00 3.00 7.00 49.15 Not allocated 11.34 11.34 Subtotal - new projects (estimate) 11.00 37.03 13.00 3.00 0.00 0.00 8.80 72.83 Total 52.53 118.21 58.00 10.60 12.00 1.68 31.48 284.50 RFT = Rain Forest Trust Fund, a multilateral fund from a number of donors, administered by The World Bank. -

Conservation Status of the Order Rodentia of Brazil: Taxonomic And
Bol. Mus. Para. Emílio Goeldi. Cienc. Nat., Belém, v. 15, n. 3, p. 535-556, set.-dez. 2020 Conservation status of the order Rodentia of Brazil: taxonomic and biogeographical patterns Estado de conservação da ordem Rodentia do Brasil: padrões taxonômicos e biogeográficos Thomas E. Lacher Jr.I, II | Shelby D. McCayI, III | Gledson Vigiano BianconiIV, V | Lilianna K. WolfI | Nicolette S. RoachI, II | Alexandre R. PercequilloVI ITexas A&M University. Department of Ecology and Conservation Biology. College Station, Texas, USA IIGlobal Wildlife Conservation. Austin, Texas, USA IIITexas A&M AgriLife Research. Natural Resources Institute. College Station, Texas, USA IVInstituto Federal do Paraná. Pinhais, Paraná, Brasil VNeotropical Institute. Research and Conservation. Curitiba, Paraná, Brasil VIUniversidade de São Paulo. Escola Superior de Agricultura “Luiz de Queiroz”. Departamento de Ciências Biológicas. Piracicaba, São Paulo, Brasil Abstract: The Global Mammal Assessment (GMA) evaluates the risk of extinction for all species of mammals, providing important data on their status to national and global conservation agencies and conventions. We assessed all of the species of Brazilian rodents as part of the GMA activities of the International Union for the Conservation of Nature Species Survival Commission (IUCN SSC) Small Mammal Specialist Group. A total of 234 species were evaluated against the IUCN Red List Criteria and placed into one of eight categories. Although rodents do not have elevated extinction risk compared to mammals as a whole, several families of caviomorph rodents have high levels of either threat, data deficiency, or both. The family Echimyidae has a large number of species and one-third of those either are species of conservation concern or data deficient. -

Cricetidae: Sigmodontinae) with an Updated Summary of Valid Tribes and Their Generic Contents
Occasional Papers Museum of Texas Tech University Number 338 15 July 2016 DESCRIPTION OF A NEW TRIBE OF SIGMODONTINE RODENTS (CRICETIDAE: SIGMODONTINAE) WITH AN UPDATED SUMMARY OF VALID TRIBES AND THEIR GENERIC CONTENTS JORGE SALAZAR-BRAVO, ULYSES F. J. PARDIÑAS, HORACIO ZEBALLOS, AND PABLO TETA ABSTRACT We provide a formal recognition to a tribal level clade composed of Andinomys and Puno- mys, two extant sigmodontine genera consistently and repeatedly recovered in the phylogenetic analyses of molecular and morphological data. As currently recognized, this tribe is distributed in middle to high elevations in the Andes of Bolivia, Peru, northern Chile, and northwestern Argentina in habitats that range from high elevation grasslands and ecotonal areas to dry Puna. Within this new clade, Punomys appears as the more specialized member as it is fully restricted to rocky outcrops and their immediate surrounding areas at elevations above 4400 m on both sides of the Altiplano. In contrast, Andinomys occupies a broad elevational range (500–4000 m) and multiple habitats, from subtropical mountain forests and semiarid Puna and Prepuna to high altitudinal grasslands. Both taxa share a number of possible synapomorphies (e.g., presence of caudal enlargement of the post-zygapophysis in the second and eighth thoracic vertebrates, unilocular-hemiglandular stomachs with a large corpus and deep incisura angularis, and very similar chromosomal complements) and other diagnostic morphological features. The supratribal phylogenetic relationships of the taxon here named are not resolved even with the moderate amount of molecular data now available. In addition, we present a revised classification for the Sigmodontinae and comment on the content and context of this unique radiation of the Cricetidae. -

Cómo Citar El Artículo Número Completo Más Información Del
Mastozoología Neotropical ISSN: 0327-9383 ISSN: 1666-0536 [email protected] Sociedad Argentina para el Estudio de los Mamíferos Argentina Brandão, Marcus Vinicius; Terra Garbino, Guilherme Siniciato; Fernandes Semedo, Thiago Borges; Feijó, Anderson; Oliveira do Nascimento, Fabio; Fernandes- Ferreira, Hugo; Vieira Rossi, Rogério; Dalponte, Julio; Carmignotto, Ana Paula MAMMALS OF MATO GROSSO, BRAZIL: ANNOTATED SPECIES LIST AND HISTORICAL REVIEW Mastozoología Neotropical, vol. 26, núm. 2, 2019, Julio-, pp. 263-306 Sociedad Argentina para el Estudio de los Mamíferos Tucumán, Argentina Disponible en: http://www.redalyc.org/articulo.oa?id=45763089010 Cómo citar el artículo Número completo Sistema de Información Científica Redalyc Más información del artículo Red de Revistas Científicas de América Latina y el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto Mastozoología Neotropical, 26(2):263-307 Mendoza, 2019 Copyright © SAREM, 2019 Versión on-line ISSN 1666-0536 hp://www.sarem.org.ar hps://doi.org/10.31687/saremMN.19.26.2.0.03 hp://www.sbmz.org Artículo MAMMALS OF MATO GROSSO, BRAZIL: ANNOTATED SPECIES LIST AND HISTORICAL REVIEW Marcus Vinicius Brandão1, Guilherme Siniciato Terra Garbino2, Thiago Borges Fernandes Semedo3,4, Anderson Feijó5, Fabio Oliveira do Nascimento1, Hugo Fernandes-Ferreira6, Rogério Vieira Rossi3, Julio Dalponte7 and Ana Paula Carmignotto8 1Mastozoologia, Museu de Zoologia da Universidade de São Paulo, 04263-000, São Paulo, SP, Brazil. [Correspondence: Marcus Vinicius Brandão <[email protected]>] 2Programa de Pós-Graduação em Zoologia, Laboratório de Mastozoologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Campus Pampulha, Belo Horizonte, MG, Brazil. -

Phylogenetic Analysis of Sigmodontine Rodents (Muroidea), with Special Reference to the Akodont Genus Deltamys
Mamm. biol. 68 (2003) 351±364 Mammalian Biology ã Urban & Fischer Verlag http://www.urbanfischer.de/journals/mammbiol Zeitschrift fuÈr SaÈ ugetierkunde Original investigation Phylogenetic analysis of sigmodontine rodents (Muroidea), with special reference to the akodont genus Deltamys By G. D'ELIÂA,E.M.GONZAÂLEZ, and U. F. J. PARDINÄ AS The University of Michigan Museum of Zoology, Ann Arbor, Michigan, USA, Laboratorio de EvolucioÂn, Facultad de Ciencias, Montevideo, Uruguay, Museo Nacional de Historia Natural, Montevideo, Uruguay and Centro Nacional Pa- tagoÂnico, Puerto Madryn, Chubut, Argentina Receipt of Ms. 24. 06. 2002 Acceptance of Ms. 13. 01. 2003 Abstract We present a comprehensive phylogenetic analysis based on cytochrome b gene sequences of sig- modontine rodents. Our particular interest is to estimate the phyletic position of Deltamys, a tax- on endemic to a small portion of the La Plata river basin in Argentina, Brazil, and Uruguay, and to assess its generic status. The three primary conclusions derived from our analyses are: (1) cotton rats (Sigmodon) are the sister group of the remaining sigmodontines, (2) the tribe Akodontini is monophyletic with moderate support, and (3) Deltamys falls outside of a clade containing all spe- cies of subgenera of Akodon yet examined, and thus we grant Deltamys status of full genus. Key words: Akodon, Akodontini, Muroidea, Sigmodontinae, phylogeny Introduction The high diversity of muroid rodents to Thomas (1916, 1918) who recognised the belonging to the New World subfamily Sig- morphologic similarity among Akodon and modontinae has seriously challenged re- some other taxa ranked as genera by him searchers attempting to understand their (e. -

Paulo Tomasi Sarti
UNIVERSIDADE DO VALE DO RIO DOS SINOS – UNISINOS PROGRAMA DE PÓS -GRADUAÇÃO EM BIOLOGIA : DIVERSIDADE E MANEJO DE VIDA SILVESTRE TESE DE DOUTORADO CONTRIBUIÇÕES À SISTEMÁTICA E DISTRIBUIÇÃO PREDITIVA DOS ROEDORES JULIOMYS (C RICETIDEA , SIGMODONTINAE ) PAULO TOMASI SARTI PROFA . ORIENTADORA : DRA . LARISSA ROSA DE OLIVEIRA PROF . CO-ORIENTADOR : DR . ALEXANDRE UARTH CHRISTOFF SÃO LEOPOLDO , JANEIRO DE 2016 PAULO TOMASI SARTI CONTRIBUIÇÕES À SISTEMÁTICA E DISTRIBUIÇÃO PREDITIVA DOS ROEDORES JULIOMYS (C RICETIDEA , SIGMODONTINAE ) Tese apresentada ao Programa de Pós- Graduação em Biologia para obtenção do título de Doutor em Biologia Área de concentração: Biologia –Diversidade e Manejo de Vida Silvestre Orientadora: Dra. Larissa Rosa de Oliveira Co-orientador: Dr. Alexandre Uarth Christoff SÃO LEOPOLDO , JANEIRO DE 2016 S249c Sarti, Paulo Tomasi Contribuições à sistemática e distribuição preditiva dos roedores Juliomys (Cricetidea, Sigmodontinae) / por Paulo Tomasi Sarti. – 2016. 160 f.: il., 30 cm. Tese (doutorado) — Universidade do Vale do Rio dos Sinos, Programa de Pós-Graduação em Biologia, 2016. Orientação: Profa. Dra. Larissa Rosa de Oliveira ; Coorientação: Prof. Dr. Alexandre Uarth Christoff. 1. Roedores. 2. Sigmodontinae . 3. Cricetidea. 4. Juliomys . 5. Morfologia. I. Título. CDU 599.32 Catalogação na Fonte: Bibliotecária Vanessa Borges Nunes - CRB 10/1556 “Daria tudo que sei pela metade do que ignoro.” René Descartes ÍNDICE 1. AGRADECIMENTOS ........................................................................................... -

Species Richness and Distribution of Neotropical Rodents, with Conservation Implications
DOI 10.1515/mammalia-2012-0050 Mammalia 2013; 77(1): 1–19 Giovanni Amori *, Federica Chiozza , Bruce D. Patterson , Carlo Rondinini , Jan Schipper and Luca Luiselli Species richness and distribution of Neotropical rodents, with conservation implications Abstract: The correlates of species richness and conser- Carlo Rondinini: Department of Biology and Biotechnology ‘ Charles vation status of South American rodents were studied Darwin ’ , Viale dell ’ Universit à 32, 00185 Rome , Italy by analyzing the ranges of 791 species (belonging to 159 Jan Schipper: Big Island Invasive Species Committee , 23 East Kawili Street, Hilo, HI 96720 , USA genera and 16 families). The distribution data (size of Luca Luiselli: Centre of Environmental Studies Demetra s.r.l. , 2 each species ’ range in km ) and the relative quantity of Eni Spa Environmental Department, via Olona 7, 00198 Rome , Italy each macrohabitat type (in km 2 ) were obtained from the Global Mammal Assessment data bank of the Interna- tional Union for Conservation of Nature (IUCN), and the Global Land Cover 2000, respectively. We excluded mainly Introduction island species from analyses but included those species that occur on both islands and the mainland. Habitats Macroecological spatial diversity patterns are among were grouped according to seven categories (shrubland, the most intriguing issues in modern ecology and bio- forest, grassland, savannah, wetlands, desert, and artifi- geography theories (e.g., Lennon et al. 2001 , Koleff cial). Mean range sizes varied significantly among fami- et al. 2003a ). Hence, ecologists have spent considerable lies, with members of the family Cuniculidae having larger effort in distinguishing between different components ranges than the species belonging to the rest of the fami- of species diversity, including alpha or local diversity lies. -

Zoology Publications 1 Fieldiana: Zoology Pub
Zoology Publications 1 Fieldiana: Zoology Pub. No. Volume 1 (Complete in Eighteen Numbers) 5 No. 1. On the Structure and Development of the Vertebral Column of Amia. By O.P. Hay. 1895. 54 pages, 3 color illus. 7 No. 2. On Certain Portions of the Skeleton of Protostega gigas. By O.P. Hay. 1895. 8 pages, 2 illus. 11 No. 3. On Sundry Collections of Mammals Received by the Field Columbian Museum from Different Localities, with Descriptions of Supposed New Species and Sub-Species. By D. G. Elliot. 1896. 18 pages, 10 illus. 12-13 Nos. 4 and 5. On Some Collections of Fishes Made in the Kankakee and Illinois Rivers. By O.P. Hay. 1896. 16 pages. On the Skeleton of Toxochelys latiremus. By O.P. Hay. 1896. 8 pages, 2 illus. 19-20 Nos. 6 and 7. List of Mammals from Somali-Land Obtained by the Museum’s East African Expedition. By D.G. Elliot. 1897. 49 pages. Remarks upon Two Species of Deer of the Genus Cervus from the Philippine Archipelago. By D.G. Elliot. 1897. 2 pages, 24 illus. 22 No. 8. List of Fishes and Reptiles Obtained by Field Columbian Museum East African Expedition to Somali-Land in 1896. By S.E. Meek. 1897. 24 pages, 2 illus. 26 No. 9. List of Collection of Shells from the Gulf of Aden, Obtained by the Museum’s East African Expedition. By W.H. Dall. 1898. 6 pages. 27 No. 10. List of Species of Mammals, Principally Rodents, Obtained by W.W. Price, Dr. S.E. Meek, G.K. -

A Remarkable New Mouse (Muridae: Sigmodontinae) from Southeastern Peru: with Comments on the Affinities of Rhagomys Rufescens (Thomas, 1886)
A Remarkable New Mouse (Muridae: Sigmodontinae) from Southeastern Peru: With Comments on the Affinities of Rhagomys rufescens (Thomas, 1886) Lucia Luna Bruce D. Patterson Abstract Field surveys of the Manu Biosphere Reserve in southeastern Peru between 1999 and 2001 secured specimens of a remarkable new species of mouse (Rodentia: Sigmodontinae). Curi- ously, this mouse shares numerous traits with the enigmatic Brazilian mouse Rhagomys rufes- cens (Thomas, 1886), known only from a pair of fragmentary specimens collected in Atlantic forest habitats during the 19th century. The position of Rhagomys within the Sigmodontinae radiation has been incertae sedis, owing both to its unique features and the limited materials for phylogenetic analysis. Here we provide a diagnosis and description of the new mouse and assess its relationships to Rhagomys rufescens on the basis of morphology; ongoing studies will shed more light on its phylogenetic relationships using morphological and genetic data in a complete phylogenetic analysis. This newly identified species widens the distribution of the genus in a disjunct pattern of distribution in South America. As recently suggested by Smith and Patton (1999), the genus Rhagomys may represent an independent lineage of Neotropical sigmodontines, one perhaps deserving of tribal recognition. Resumen Evaluaciones de campo en la Reserva de Biosfera de Manu en el sureste de Peru durante 1999 y 2001 resultaron dentro en otros, en la colecta de tres especimenes de una remarcable nueva especie de raton (Rodentia: Sigmodontinae). Curiosamente, este raton comparte nume- rosas caracteristicas con el enigmatico raton brasilero Rhagomys rufescens (Thomas, 1886) conocido solamente de un par de especimenes incompletos colectados en la Mata Atlantica durante el siglo XIX.