Redalyc.First Record of Rhagomys (Mammalia: Sigmodontinae) in Bolivia
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Rodentia: Cricetidae: Sigmodontinae) in São Paulo State, Southeastern Brazil: a Locally Extinct Species?
Volume 55(4):69‑80, 2015 THE PRESENCE OF WILFREDOMYS OENAX (RODENTIA: CRICETIDAE: SIGMODONTINAE) IN SÃO PAULO STATE, SOUTHEASTERN BRAZIL: A LOCALLY EXTINCT SPECIES? MARCUS VINÍCIUS BRANDÃO¹ ABSTRACT The Rufous-nosed Mouse Wilfredomys oenax is a rare Sigmodontinae rodent known from scarce records from northern Uruguay and south and southeastern Brazil. This species is under- represented in scientific collections and is currently classified as threathened, being considered extinct at Curitiba, Paraná, the only confirmed locality of the species at southeastern Brazil. Although specimens from São Paulo were already reported, the presence of this species in this state seems to have passed unnoticed in recent literature. Through detailed morphological ana- lyzes of specimens cited in literature, the present work confirms and discusses the presence of this species in São Paulo state from a specimen collected more than 70 years ago. Recently, by the use of modern sampling methods, other rare Sigmodontinae rodents, such as Abrawayomys ruschii, Phaenomys ferrugineous and Rhagomys rufescens, have been recorded to São Paulo state. However, no specimen of Wilfredomys oenax has been recently reported indicating that this species might be locally extinct. The record mentioned here adds another species to the state of São Paulo mammal diversity and reinforces the urgency of studying Wilfredomys oenax. Key-Words: Atlantic Forest; Scientific collection; Threatened species. INTRODUCTION São Paulo is one the most studied states in Brazil regarding to fauna. Mammal lists from this state have Mammal species lists based on voucher-speci- been elaborated since the late XIX century (Von Iher- mens and literature records are essential for offering ing, 1894; Vieira, 1944a, b, 1946, 1950, 1953; Vivo, groundwork to understand a species distribution and 1998). -

Pontificia Universidad Católica Del Ecuador
CORE Metadata, citation and similar papers at core.ac.uk Provided by Repositorio Digital PUCE PONTIFICIA UNIVERSIDAD CATÓLICA DEL ECUADOR FACULTAD DE CIENCIAS EXACTAS Y NATURALES ESCUELA DE BIOLOGÍA Genetic and morphological variability of the páramo Oldfield mouse Thomasomys paramorum Thomas, 1898 (Rodentia: Cricetidae): evidence for a complex of species Tesis previa a la obtención del título de Magister en Biología de la Conservación CARLOS ESTEBAN BOADA TERÁN Quito, 2013 II Certifico que la Tesis de Maestría en Biología de la Conservación del candidato Carlos Esteban Boada Terán ha sido concluida de conformidad con las normas establecidas; por tanto, puede ser presentada para la calificación correspondiente. Dr. Omar Lenin Torres Carvajal Director de Tesis Mayo de 2013 III Dedicado a mi hijo Joaquín IV ACKNOWLEDGMENTS This manuscript was presented as a requirement for graduation at Pontificia Universidad Católica del Ecuador, Master´s program in Conservation Biology. I thank O. Torres- Carvajal for his mentorship, J. Patton for reviewing the first draft of the manuscript and provide valuable suggestions and comments, and S. Burneo for allowing the examination of specimens deposited at Museo de Zoología (QCAZ), sección Mastozoología. For field support I thank Viviana Narváez, Daniel Chávez, Roberto Carrillo, Simón Lobos, Julia Salvador, Adriana Argoti and Amy Scott. Finally I am grateful to Mary Eugenia Ordóñez, Gaby Nichols, Andrea Manzano and Diana Flores for their help in the laboratory. I thank to SENESCYT because most laboratory equipment was purchased with the project "Inventory and Morphological Characterization and Genetic Diversity of Amphibians, Reptiles and Birds of the Andes of Ecuador", code PIC-08-0000470. -

Proquest Dissertations
The Neotropical rodent genus Rhipidom ys (Cricetidae: Sigmodontinae) - a taxonomic revision Christopher James Tribe Thesis submitted for the degree of Doctor of Philosophy University College London 1996 ProQuest Number: 10106759 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest. ProQuest 10106759 Published by ProQuest LLC(2016). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. Microform Edition © ProQuest LLC. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 ABSTRACT South American climbing mice and rats, Rhipidomys, occur in forests, plantations and rural dwellings throughout tropical South America. The genus belongs to the thomasomyine group, an informal assemblage of plesiomorphous Sigmodontinae. Over 1700 museum specimens were examined, with the aim of providing a coherent taxonomic framework for future work. A shortage of discrete and consistent characters prevented the use of strict cladistic methodology; instead, morphological assessments were supported by multivariate (especially principal components) analyses. The morphometric data were first assessed for measurement error, ontogenetic variation and sexual dimorphism; measurements with most variation from these sources were excluded from subsequent analyses. The genus is characterized by a combination of reddish-brown colour, long tufted tail, broad feet with long toes, long vibrissae and large eyes; the skull has a small zygomatic notch, squared or ridged supraorbital edges, large oval braincase and short palate. -

Chec List ISSN 1809-127X (Available at Journal of Species Lists and Distribution
Check List 8(3): 557-559, 2012 © 2012 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution N Rhagomys rufescens Sigmodontinae) Thomas, 1886 ISTRIBUTIO New distribution reports of (Rodentia: D * and Maria Rita Silvério Pires RAPHIC G Caryne Aparecida de Carvalho Braga EO G N Universidade Federal de Ouro Preto, Departamento de Evolução, Biodiversidade e Meio Ambiente, Laboratório de Zoologia dos Vertebrados, O [email protected] Campus Morro do Cruzeiro. CEP 35400-000. Ouro Preto, MG, Brasil. * Corresponding author. E-mail: OTES N Abstract: Rhagomys rufescens is a rare, arboreal sigmodontine rodent endemic to the Brazilian Atlantic Forest biome. This species is known from eight localities in Brazil. Here we present a new report based on four individuals of this species registered in Serra do Ouro Branco, municipality of Ouro Branco (Minas Gerais, Brazil). One juvenile male, one adult male ofand the two last juvenile registered females location. were captured in pitfall traps during the rainy season, in a study of small mammal ecology. This is the first record for the Espinhaço Mountain range and the northernmost report for the species in this state, 85 km northeast Rhagomys rufescens (Thomas, 1886) is an enigmatic level as “incertae sedis” (Reig 1980; 1984; McKenna and Forest domain, at the south of the Espinhaço mountain arboreal sigmodontine rodent classified at the suprageneric range. Our report occurred in an area of vegetation characterized as semi-deciduous mountain forest. A Bell 1997; Smith and Patton 1999; Musser and Carleton portion of the sampled locality is now inside a conservation 2005). -

DE Thomasomys Y DESCRIPCIÓN DE UNA NUEVA ESPECIE (RODENTIA, CRICETIDAE)
Mastozoología Neotropical, 26(2):308-330 Mendoza, 2019 Copyright © SAREM, 2019 Versión on-line ISSN 1666-0536 hp://www.sarem.org.ar hps://doi.org/10.31687/saremMN.19.26.2.0.04 hp://www.sbmz.org Artículo DIVERSIDAD INSOSPECHADA EN LOS ANDES DE ECUADOR: FILOGENIA DEL GRUPO “cinereus” DE Thomasomys Y DESCRIPCIÓN DE UNA NUEVA ESPECIE (RODENTIA, CRICETIDAE) Jorge Brito1, Nicolás Tinoco2, Jenny Curay1, Rocío Vargas1, Carolina Reyes-Puig3, Víctor Romero4,5 y Ulyses F. J. Pardiñas1, 6 1 Instituto Nacional de Biodiversidad (INABIO), Quito, Ecuador. [Correspondencia: Jorge Brito <[email protected]>] 2 Museo de Zoología, Escuela de Biología, Ponticia Universidad Católica del Ecuador, Quito, Ecuador. 3 Instituto de Zoología Terrestre, Museo de Zoología, Instituto BIOSFERA, Colegio de Ciencias Biológicas y Ambientales, Universidad San Francisco de Quito (USFQ) Cumbayá, Quito, Ecuador. 4 Museo de Zoología, Universidad Técnica Particular de Loja, San Cayetano Alto, Loja, Ecuador. 5 Postgrado en Ciencias Biológicas, Departamento de Estudios Ambientales, Universidad Simón Bolívar, Caracas, Venezuela. 6 Instituto de Diversidad y Evolución Austral (IDEAus – CONICET), Puerto Madryn, Chubut, Argentina. RESUMEN. Con base en ejemplares recolectados en los Andes surorientales de Ecuador, Parque Nacional Sangay, pero también integrando otros materiales de colecciones, se efectuó una revisión del grupo “cinereus” del género de cricétidos Thomasomys Coues, 1884 (Sigmodontinae, Thomasomyini). Como resultado de dicha revisión, además de proponerse una hipótesis -
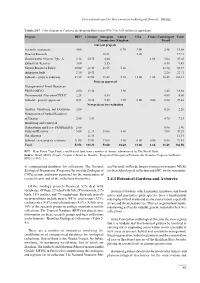
CBD First National Report
First national report for the Convention on Biological Diversity - BRAZIL Tabela 2-37. Pilot Program to Conserve the Brazilian Rain Forest PPG-7 (In US$ million or equivalent). Projects RFT* Germany European United USA France Counterpart Total Commission Kingdom Brazil Current projects Scientific institutions 9.00 0.70 3.00 2.98 15.68 Directed Research 10.91 9.00 19.91 Demonstrative Projects Type A 3.18 20.75 4.44 1.68 3.00 33.05 Extractivist Reserves 3.00 5.55 0.90 9.45 Natural Resources Policy 20.00 28.48 18.55 5.00 11.40 83.43 Indigenous lands 2.10 18.41 2.20 22.71 Subtotal – projects underway 37.28 67.64 39.45 5.70 12.00 1.68 20.48 184.23 Projects approved Management of Forest Resources- PROMANEJO 2.00 13.54 1.90 1.40 18.84 Environmental Education-CEDUC 2.25 5.55 0.80 8.60 Subtotal - projects approved 4.25 13.54 5.55 1.90 0.00 0.00 2.20 27.44 New projects for evaluation Analysis, Monitoring and Evaluation 2.00 0.20 2.20 Management of Natural Resources of Várzeas 2.00 4.54 0.70 7.24 Monitoring and Control of Deforestation and Fires- PRODESQUE 2.00 0.90 2.90 Parks and Reserves 5.00 21.15 13.00 3.00 7.00 49.15 Not allocated 11.34 11.34 Subtotal - new projects (estimate) 11.00 37.03 13.00 3.00 0.00 0.00 8.80 72.83 Total 52.53 118.21 58.00 10.60 12.00 1.68 31.48 284.50 RFT = Rain Forest Trust Fund, a multilateral fund from a number of donors, administered by The World Bank. -

Universidad Central Del Ecuador
UNIVERSIDAD CENTRAL DEL ECUADOR FACULTAD DE CIENCIAS BIOLÓGICAS CARRERA DE CIENCIAS BIOLÓGICAS Y AMBIENTALES ÁREAS PRIORITARIAS PARA LA CONSERVACIÓN Y ZONAS DE ENDEMISMO DEL GÉNERO Thomasomys EN ECUADOR Autor: Moscoso Ruales María Gabriela Tutor: Msc. Byron Daniel Medina Torres Quito, febrero 2019 UNIVERSIDAD CENTRAL DEL ECUADOR FACULTAD DE CIENCIAS BIOLÓGICAS CARRERA DE CIENCIAS BIOLÓGICAS Y AMBIENTALES Áreas prioritarias para la conservación y zonas de endemismo del género Thomasomys en Ecuador. Trabajo de titulación presentado como requisito previo a la obtención del Título de Licenciada en Ciencias Biológicas y Ambientales Autor: Moscoso Ruales María Gabriela Tutor: Msc. Byron Daniel Medina Torres Quito, febrero 2019 ii DERECHOS DE AUTOR Yo, María Gabriela Moscoso Ruales en calidad de autora y titulares de los derechos morales y patrimoniales del trabajo de titulación Áreas prioritarias para la conservación y zonas de endemismo del género Thomasomys en Ecuador, modalidad Proyecto de Grado, de conformidad con el Art. 114 del CÓDIGO ORGÁNICO DE LA ECONOMÍA SOCIAL DE LOS CONOCIMIENTOS, CREATIVIDAD E INNOVACIÓN, concedemos a favor de la Universidad Central del Ecuador una licencia gratuita, intransferible y no exclusiva para el uso no comercial de la obra, con fines estrictamente académicos. Conservamos a mi/nuestro favor todos los derechos de autor sobre la obra, establecidos en la normativa citada. Así mismo, autorizo/autorizamos a la Universidad Central del Ecuador para que realice la digitalización y publicación de este trabajo de titulación en el repositorio virtual, de conformidad a lo dispuesto en el Art. 144 de la Ley Orgánica de Educación Superior. El (los) autor (es) declara (n) que la obra objeto de la presente autorización es original en su forma de expresión y no infringe el derecho de autor de terceros, asumiendo la responsabilidad por cualquier reclamación que pudiera presentarse por esta causa y liberando a la Universidad de toda responsabilidad. -

First Record of Thomasomys Cinereusthomas, 1882
THERYA, 2020, Vol. 11 (1): 41-45 DOI: 10.12933/therya-20-640 ISSN 2007-3364 First record of Thomasomys cinereus Thomas, 1882 (Rodentia, Cricetidae, Sigmodontinae) in Ecuador PABLO A. MORENO CÁRDENAS1, 2* AND MARYLIN NOVILLO-GONZALEZ3 1 Rumipamba No. 341 y Av. De los Shyris, Sección de Mastozoología, Instituto Nacional de Biodiversidad del Ecuador (INABIO) BOX 170515, Pichincha. Quito, Ecuador. Email: [email protected] (PAMC). 2 Mastozoología del Departamento de Ciencias Biológicas de la Escuela Politécnica Nacional (PAMC). Avenida Ladrón de Guevara E-11 253 e Isabel la Católica. Apartado 17-012759. Pichincha. Quito, Ecuador. 3 Department of Biology and Marine Biology, University of North Carolina Wilmington UNCW, Wilmington. North Carolina, U.S.A. Email: [email protected] (MN-G). *Corresponding author The Andean forests of southwestern Ecuador and northern Peru, hold a unique assemblage of species, given the existence of high habitat diversity. Our aim is to characterize the small mammal diversity at Yacuri National Park in southern Ecuador. We collected small non-volant mammals, using Sherman, Pitfall, and Tomahawk traps, in wet and dry habitats in the Yacuri National Park; and obtained some specimens of rodents and marsupials in the pluviseasonal forest in southern Ecuador. We registered several non-volant small mammal species, including the first Ecuadorian record of the olive-gray mouse (Thomasomys cinereus). This record expands the known species distribution, 10 km from the northernmost location in Peru within the same ecosystems. Los bosques de los Andes del suroccidente de Ecuador y norte de Perú, comparten una diversidad única en especies de mamíferos, debido a la amplia diversidad de hábitats. -

Conservation Status of the Order Rodentia of Brazil: Taxonomic And
Bol. Mus. Para. Emílio Goeldi. Cienc. Nat., Belém, v. 15, n. 3, p. 535-556, set.-dez. 2020 Conservation status of the order Rodentia of Brazil: taxonomic and biogeographical patterns Estado de conservação da ordem Rodentia do Brasil: padrões taxonômicos e biogeográficos Thomas E. Lacher Jr.I, II | Shelby D. McCayI, III | Gledson Vigiano BianconiIV, V | Lilianna K. WolfI | Nicolette S. RoachI, II | Alexandre R. PercequilloVI ITexas A&M University. Department of Ecology and Conservation Biology. College Station, Texas, USA IIGlobal Wildlife Conservation. Austin, Texas, USA IIITexas A&M AgriLife Research. Natural Resources Institute. College Station, Texas, USA IVInstituto Federal do Paraná. Pinhais, Paraná, Brasil VNeotropical Institute. Research and Conservation. Curitiba, Paraná, Brasil VIUniversidade de São Paulo. Escola Superior de Agricultura “Luiz de Queiroz”. Departamento de Ciências Biológicas. Piracicaba, São Paulo, Brasil Abstract: The Global Mammal Assessment (GMA) evaluates the risk of extinction for all species of mammals, providing important data on their status to national and global conservation agencies and conventions. We assessed all of the species of Brazilian rodents as part of the GMA activities of the International Union for the Conservation of Nature Species Survival Commission (IUCN SSC) Small Mammal Specialist Group. A total of 234 species were evaluated against the IUCN Red List Criteria and placed into one of eight categories. Although rodents do not have elevated extinction risk compared to mammals as a whole, several families of caviomorph rodents have high levels of either threat, data deficiency, or both. The family Echimyidae has a large number of species and one-third of those either are species of conservation concern or data deficient. -

Cricetidae: Sigmodontinae) with an Updated Summary of Valid Tribes and Their Generic Contents
Occasional Papers Museum of Texas Tech University Number 338 15 July 2016 DESCRIPTION OF A NEW TRIBE OF SIGMODONTINE RODENTS (CRICETIDAE: SIGMODONTINAE) WITH AN UPDATED SUMMARY OF VALID TRIBES AND THEIR GENERIC CONTENTS JORGE SALAZAR-BRAVO, ULYSES F. J. PARDIÑAS, HORACIO ZEBALLOS, AND PABLO TETA ABSTRACT We provide a formal recognition to a tribal level clade composed of Andinomys and Puno- mys, two extant sigmodontine genera consistently and repeatedly recovered in the phylogenetic analyses of molecular and morphological data. As currently recognized, this tribe is distributed in middle to high elevations in the Andes of Bolivia, Peru, northern Chile, and northwestern Argentina in habitats that range from high elevation grasslands and ecotonal areas to dry Puna. Within this new clade, Punomys appears as the more specialized member as it is fully restricted to rocky outcrops and their immediate surrounding areas at elevations above 4400 m on both sides of the Altiplano. In contrast, Andinomys occupies a broad elevational range (500–4000 m) and multiple habitats, from subtropical mountain forests and semiarid Puna and Prepuna to high altitudinal grasslands. Both taxa share a number of possible synapomorphies (e.g., presence of caudal enlargement of the post-zygapophysis in the second and eighth thoracic vertebrates, unilocular-hemiglandular stomachs with a large corpus and deep incisura angularis, and very similar chromosomal complements) and other diagnostic morphological features. The supratribal phylogenetic relationships of the taxon here named are not resolved even with the moderate amount of molecular data now available. In addition, we present a revised classification for the Sigmodontinae and comment on the content and context of this unique radiation of the Cricetidae. -

Cómo Citar El Artículo Número Completo Más Información Del
Mastozoología Neotropical ISSN: 0327-9383 ISSN: 1666-0536 [email protected] Sociedad Argentina para el Estudio de los Mamíferos Argentina Brandão, Marcus Vinicius; Terra Garbino, Guilherme Siniciato; Fernandes Semedo, Thiago Borges; Feijó, Anderson; Oliveira do Nascimento, Fabio; Fernandes- Ferreira, Hugo; Vieira Rossi, Rogério; Dalponte, Julio; Carmignotto, Ana Paula MAMMALS OF MATO GROSSO, BRAZIL: ANNOTATED SPECIES LIST AND HISTORICAL REVIEW Mastozoología Neotropical, vol. 26, núm. 2, 2019, Julio-, pp. 263-306 Sociedad Argentina para el Estudio de los Mamíferos Tucumán, Argentina Disponible en: http://www.redalyc.org/articulo.oa?id=45763089010 Cómo citar el artículo Número completo Sistema de Información Científica Redalyc Más información del artículo Red de Revistas Científicas de América Latina y el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto Mastozoología Neotropical, 26(2):263-307 Mendoza, 2019 Copyright © SAREM, 2019 Versión on-line ISSN 1666-0536 hp://www.sarem.org.ar hps://doi.org/10.31687/saremMN.19.26.2.0.03 hp://www.sbmz.org Artículo MAMMALS OF MATO GROSSO, BRAZIL: ANNOTATED SPECIES LIST AND HISTORICAL REVIEW Marcus Vinicius Brandão1, Guilherme Siniciato Terra Garbino2, Thiago Borges Fernandes Semedo3,4, Anderson Feijó5, Fabio Oliveira do Nascimento1, Hugo Fernandes-Ferreira6, Rogério Vieira Rossi3, Julio Dalponte7 and Ana Paula Carmignotto8 1Mastozoologia, Museu de Zoologia da Universidade de São Paulo, 04263-000, São Paulo, SP, Brazil. [Correspondence: Marcus Vinicius Brandão <[email protected]>] 2Programa de Pós-Graduação em Zoologia, Laboratório de Mastozoologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Campus Pampulha, Belo Horizonte, MG, Brazil. -

List of Taxa for Which MIL Has Images
LIST OF 27 ORDERS, 163 FAMILIES, 887 GENERA, AND 2064 SPECIES IN MAMMAL IMAGES LIBRARY 31 JULY 2021 AFROSORICIDA (9 genera, 12 species) CHRYSOCHLORIDAE - golden moles 1. Amblysomus hottentotus - Hottentot Golden Mole 2. Chrysospalax villosus - Rough-haired Golden Mole 3. Eremitalpa granti - Grant’s Golden Mole TENRECIDAE - tenrecs 1. Echinops telfairi - Lesser Hedgehog Tenrec 2. Hemicentetes semispinosus - Lowland Streaked Tenrec 3. Microgale cf. longicaudata - Lesser Long-tailed Shrew Tenrec 4. Microgale cowani - Cowan’s Shrew Tenrec 5. Microgale mergulus - Web-footed Tenrec 6. Nesogale cf. talazaci - Talazac’s Shrew Tenrec 7. Nesogale dobsoni - Dobson’s Shrew Tenrec 8. Setifer setosus - Greater Hedgehog Tenrec 9. Tenrec ecaudatus - Tailless Tenrec ARTIODACTYLA (127 genera, 308 species) ANTILOCAPRIDAE - pronghorns Antilocapra americana - Pronghorn BALAENIDAE - bowheads and right whales 1. Balaena mysticetus – Bowhead Whale 2. Eubalaena australis - Southern Right Whale 3. Eubalaena glacialis – North Atlantic Right Whale 4. Eubalaena japonica - North Pacific Right Whale BALAENOPTERIDAE -rorqual whales 1. Balaenoptera acutorostrata – Common Minke Whale 2. Balaenoptera borealis - Sei Whale 3. Balaenoptera brydei – Bryde’s Whale 4. Balaenoptera musculus - Blue Whale 5. Balaenoptera physalus - Fin Whale 6. Balaenoptera ricei - Rice’s Whale 7. Eschrichtius robustus - Gray Whale 8. Megaptera novaeangliae - Humpback Whale BOVIDAE (54 genera) - cattle, sheep, goats, and antelopes 1. Addax nasomaculatus - Addax 2. Aepyceros melampus - Common Impala 3. Aepyceros petersi - Black-faced Impala 4. Alcelaphus caama - Red Hartebeest 5. Alcelaphus cokii - Kongoni (Coke’s Hartebeest) 6. Alcelaphus lelwel - Lelwel Hartebeest 7. Alcelaphus swaynei - Swayne’s Hartebeest 8. Ammelaphus australis - Southern Lesser Kudu 9. Ammelaphus imberbis - Northern Lesser Kudu 10. Ammodorcas clarkei - Dibatag 11. Ammotragus lervia - Aoudad (Barbary Sheep) 12.