And Targeted Axillary Dissection (TAD) After Neoadjuvant Chemotherapy (NACT) for Node-Positive Breast Cancer: Systematic Review and Pooled Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Follicular Lymphoma
Follicular Lymphoma What is follicular lymphoma? Let us explain it to you. www.anticancerfund.org www.esmo.org ESMO/ACF Patient Guide Series based on the ESMO Clinical Practice Guidelines FOLLICULAR LYMPHOMA: A GUIDE FOR PATIENTS PATIENT INFORMATION BASED ON ESMO CLINICAL PRACTICE GUIDELINES This guide for patients has been prepared by the Anticancer Fund as a service to patients, to help patients and their relatives better understand the nature of follicular lymphoma and appreciate the best treatment choices available according to the subtype of follicular lymphoma. We recommend that patients ask their doctors about what tests or types of treatments are needed for their type and stage of disease. The medical information described in this document is based on the clinical practice guidelines of the European Society for Medical Oncology (ESMO) for the management of newly diagnosed and relapsed follicular lymphoma. This guide for patients has been produced in collaboration with ESMO and is disseminated with the permission of ESMO. It has been written by a medical doctor and reviewed by two oncologists from ESMO including the lead author of the clinical practice guidelines for professionals, as well as two oncology nurses from the European Oncology Nursing Society (EONS). It has also been reviewed by patient representatives from ESMO’s Cancer Patient Working Group. More information about the Anticancer Fund: www.anticancerfund.org More information about the European Society for Medical Oncology: www.esmo.org For words marked with an asterisk, a definition is provided at the end of the document. Follicular Lymphoma: a guide for patients - Information based on ESMO Clinical Practice Guidelines – v.2014.1 Page 1 This document is provided by the Anticancer Fund with the permission of ESMO. -

December 2020 E-Tips Solid Tumor Rules
New Jersey State Cancer Registry December 2020 E-Tips Cancer Epidemiology Services http://www.nj.gov/health/ces (609) 633-0500 Solid Tumor Rules: December 2020 Update ICD-0-3.2 changes have also been added to applicable site modules. Most changes are minor: terminology, additional definitions, new notes and examples. In order to clarify histology coding instructions, new rules have been added and histology tables updated. These updates do not require review of already abstracted cases. The December 2020 rules replace the current rules and should be used now. SEER Strongly recommends reading the December 2020 Change Log to understand what changes were made. The updated Solid Tumor Rules may be accessed at: https://seer.cancer.gov/tools/solidtumor/ Reportability Changes for 2021 Radiation Coding Total Dose (1533) Starting 01/01/2021 the following terms are reportable: If doses across phases to a single point of region, code Sum of all phases. ** (see 2019 update below) Early or evolving melanoma in situ, or any other early or If doses are to multiple metastatic sites, code highest evolving melanoma, are reportable. dose site. If doses are to primary site and metastatic site, code dose All GIST tumors are reportable and classified as 8936/3 in from the primary site only. ICD-O-3.2. When you have two different sites, you cannot add the Nearly all thymomas are reportable; the exceptions are doses together to get the total dose. microscopic thymoma or thymoma benign (8580/0), micronodular thymoma with lymphoid stroma (8580/1), Radiation Tips and updates for 2019 and ectopic hamartomatous thymoma (8587/0). -

About Mastectomy and Sentinel Lymph Node Biopsy
All About Mastectomy and Sentinel Lymph Node Biopsy One of the most important goals of Moffitt Cancer Center is to provide you with quality patient care through education, research and patient care. The following information has been developed to help you understand the mastectomy and sentinel lymph node biopsy procedures for which you have been scheduled. Members of your health care team will review this information with you and answer any questions that you may have. Definitions: Mastectomy – Removal of the entire breast but not the muscles underneath. Lymph Nodes - Small bean shaped glands found in the armpit. They remove waste and fluids from the arm and breast and help fight infection. They are also call lymph glands. Sentinel Lymph Node - The first place breast cancer may metastasize (or spread) is to lymph nodes in the axilla (underarm area) on the side of the body where the cancer is. The first lymph node(s) are referred to as the sentinel lymph node(s) or the “gate keepers”. These nodes are first biopsied to determine if the cancer has spread. If cancer cells are not found in the sentinel lymph node(s) that were removed, it will not be necessary to remove the remaining lymph nodes in the arm pit. Sentinel Lymph Node Mapping and Biopsy - This procedure is a two step process. Performing the sentinel lymph node biopsy requires a small incision in your axilla. In the first step, the doctor injects a radioactive substance and/ or blue dye in the area around the tumor. Lymphatic channels (like blood vessels) carry these materials to the sentinel lymph node. -

Consensus Guideline on the Management of the Axilla in Patients with Invasive/In-Situ Breast Cancer
- Official Statement - Consensus Guideline on the Management of the Axilla in Patients With Invasive/In-Situ Breast Cancer Purpose To outline the management of the axilla for patients with invasive and in-situ breast cancer. Associated ASBrS Guidelines or Quality Measures 1. Performance and Practice Guidelines for Sentinel Lymph Node Biopsy in Breast Cancer Patients – Revised November 25, 2014 2. Performance and Practice Guidelines for Axillary Lymph Node Dissection in Breast Cancer Patients – Approved November 25, 2014 3. Quality Measure: Sentinel Lymph Node Biopsy for Invasive Breast Cancer – Approved November 4, 2010 4. Prior Position Statement: Management of the Axilla in Patients With Invasive Breast Cancer – Approved August 31, 2011 Methods A literature review inclusive of recent randomized controlled trials evaluating the use of sentinel lymph node surgery and axillary lymph node dissection for invasive and in-situ breast cancer as well as the pathologic review of sentinel lymph nodes and indications for axillary radiation was performed. This is not a complete systematic review but rather, a comprehensive review of recent relevant literature. A focused review of non-randomized controlled trials was then performed to develop consensus guidance on management of the axilla in scenarios where randomized controlled trials data is lacking. The ASBrS Research Committee developed a consensus document, which was reviewed and approved by the ASBrS Board of Directors. Summary of Data Reviewed Recommendations Based on Randomized Controlled -

Application of Preoperative Computed Tomographic Lymphography For
Wen et al. BMC Surg (2021) 21:187 https://doi.org/10.1186/s12893-021-01190-7 RESEARCH ARTICLE Open Access Application of preoperative computed tomographic lymphography for precise sentinel lymph node biopsy in breast cancer patients Shishuai Wen1,2,3†, Yiran Liang1†, Xiaoli Kong1, Baofeng Liu4, Tingting Ma1, Yeqing Zhou1, Liyu Jiang1, Xiaoyan Li1 and Qifeng Yang1,5,6* Abstract Background: In light of the extensive application of sentinel lymph node biopsy (SLNB) in clinically node-negative breast cancer patients and the recently investigated failure of SLNB after lumpectomy, it has become important to explore methods for preoperative mapping of sentinel lymph nodes (SLNs) and their lymphatics to direct precise SLNB and improve the identifcation rate of SLNs. Methods: Twenty-seven patients with suspected breast cancer based on the results of the clinical examination and imaging were enrolled in the study. Computed tomographic lymphography (CTLG) followed by CT three-dimensional reconstruction was performed to determine the localization of SLNs and lymphatics on the body surface preopera- tively. Intraoperatively combined staining with methylene blue and indocyanine green was used to evaluate the accuracy and feasibility of CTLG. Results: SLNs and lymphatics from the breast were identifed using CTLG in all patients, and preoperative SLNs and lymphatics localization on the body surface showed a signifcant role in the selection of operative incision and injec- tion points. The accuracy rate of SLN and lymphatic detection by CTLG was 92.6% compared with intraoperatively combined staining. Moreover, preoperative CTLG performed well in SLN number detection, and the accuracy rate was 95.2%. Conclusion: We evaluate the procedure and application of preoperative CTLG in the superfcial localization of SLNs and lymphatics, which may lead to a decreased incidence of cutting of the lymphatics of SLNs and consequently more rapid and accurate SLN detection. -

Update on Sentinel Lymph Node Biopsy in Surgical Staging of Endometrial Carcinoma
Journal of Clinical Medicine Review Update on Sentinel Lymph Node Biopsy in Surgical Staging of Endometrial Carcinoma Ane Gerda Z Eriksson 1,2,* , Ben Davidson 2,3, Pernille Bjerre Trent 1,2, Brynhildur Eyjólfsdóttir 1, Gunn Fallås Dahl 1, Yun Wang 1 and Anne Cathrine Staff 2,4 1 Department of Gynecologic Oncology, Oslo University Hospital, Norwegian Radium Hospital, N-0310 Oslo, Norway; [email protected] (P.B.T.); [email protected] (B.E.); [email protected] (G.F.D.); [email protected] (Y.W.) 2 Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, N-0316 Oslo, Norway; [email protected] (B.D.); [email protected] (A.C.S.) 3 Department of Pathology, Oslo University Hospital, Norwegian Radium Hospital, N-0310 Oslo, Norway 4 Division of Obstetrics and Gynaecology, Oslo University Hospital, Ullevål, N-0424 Oslo, Norway * Correspondence: [email protected] Abstract: Sentinel lymph node (SLN) biopsy has emerged as an alternative staging approach in women with assumed early-stage endometrial carcinoma. Through image-guided surgery and pathologic ultrastaging, the SLN approach is introducing “precision medicine” to the surgical management of gynecologic cancers, providing a comprehensive evaluation of high-yield lymph nodes. This approach improves the surgeons’ ability to detect small-volume metastatic disease while reducing intraoperative and postoperative morbidity associated with lymphadenectomy. Although the majority of clinicians in Europe and the USA have recognized the value of SLN biopsy in endometrial carcinoma and introduced this as part of clinical practice, there is ongoing debate Citation: Eriksson, A.G.Z; Davidson, regarding its role in very low-risk patients as well as in patients at high risk of nodal metastasis. -
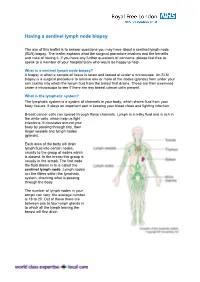
Having a Sentinel Lymph Node Biopsy
Having a sentinel lymph node biopsy The aim of this leaflet is to answer questions you may have about a sentinel lymph node (SLN) biopsy. The leaflet explains what the surgical procedure involves and the benefits and risks of having it. If you have any further questions or concerns, please feel free to speak to a member of your hospital team who would be happy to help. What is a sentinel lymph node biopsy? A biopsy is when a sample of tissue is taken and looked at under a microscope. An SLN biopsy is a surgical procedure to remove one or more of the nodes (glands) from under your arm (axilla) into which the lymph fluid from the breast first drains. These are then examined under a microscope to see if there are any breast cancer cells present. What is the lymphatic system? The lymphatic system is a system of channels in your body, which drains fluid from your body tissues. It plays an important part in keeping your blood clean and fighting infection. Breast cancer cells can spread through these channels. Lymph is a milky fluid and is rich in the white cells, which help us fight infections. It circulates around your body by passing through tiny, then larger vessels and lymph nodes (glands). Each area of the body will drain lymph fluid into certain nodes, usually to the group of nodes which is closest. In the breast this group is usually in the armpit. The first node the fluid drains in to is called the sentinel lymph node. -

Standards for Oncology Registry Entry STORE 2018
STandards for Oncology Registry Entry STORE 2018 Effective for Cases Diagnosed January 1, 2018 STORE STandards for Oncology Registry Entry Released 2018 (Incorporates all updates to Commission on Cancer, National Cancer Database Data standards since FORDS was revised in 2016) Effective for cases diagnosed January 1, 2018 See Appendix A for Updates since FORDS: Revised for 2016. Version 1.0 © 2018 AMERICAN COLLEGE OF SURGEONS All Rights Reserved STORE 2018 Table of Contents Table of Contents Table of Contents ......................................................................................................................... ii Foreword ..................................................................................................................................... 1 FROM “FORDS” TO “STORE” ..................................................................................................................... 1 Preface 2018 ................................................................................................................................ 2 Comorbidities and Complications ............................................................................................................. 2 Revisions to Staging Requirements ........................................................................................................... 2 Staging Data Items No Longer Required for Cases Diagnosed in 2018 and Later (Required for Cases Diagnosed 2017 and Earlier) ................................................................................................................ -

Autologous Peripheral Blood Stem Cell Transplantation in Children and Adolescents with Non‑Hodgkin Lymphoma
1826 ONCOLOGY LETTERS 10: 1826-1830, 2015 Autologous peripheral blood stem cell transplantation in children and adolescents with non‑Hodgkin lymphoma WEI GUI, LIPING SU, JIANXIA HE, LIEYANG WANG and TAO GUAN Department of Hematology, Shanxi Tumor Hospital, Taiyuan, Shanxi 030013, P.R. China Received June 14, 2014; Accepted April 14, 2015 DOI: 10.3892/ol.2015.3455 Abstract. The aim of this study was to evaluate the effect and (BL), anaplastic large-cell lymphoma and diffuse large safety of autologous peripheral blood stem cell transplantation B-cell lymphoma (2). Recent developments in chemotherapy (APBSCT) in children and adolescents with non-Hodgkin regimens, including BFM90, VDCLP and HyperCVAD/ lymphoma (NHL). Ten patients with NHL were analyzed MA, intrathecal methotraxate, cytarabine, dexamethasone retrospectively. In all the patients, lymph node enlargement and combined chemotherapy with rituximab for B-cell NHL was most frequently detected. Patients with a mediastinal mass patients, have rapidly improved the survival rate of pediatric presented with a cough, palpitation and shortness of breath. NHL patients (3-6). Long term event-free survival (EFS) has Extranodal patients presented with abdominal pain, inability been exhibited to be 60-90%. However, for ~10-30% of children to walk and vaginal bleeding. All patients underwent APBSCT with NHL who receive modern chemotherapy, the treatment is with conditioning regimens BEAM or BuCy. Among them, likely to be unsuccessful (7-10). Recently, autologous peripheral four patients with B-cell NHL received rituximab in addition blood stem cell transplantation (APBSCT), with conditioning to the conditioning regimen. Hematopoietic reconstitution was regimens BEAM or BuCy, has improved the long-term survival observed in all patients. -

Lymphatic Mapping with Sentinel Node Biopsy
PATIENT & CAREGIVER EDUCATION Lymphatic Mapping with Sentinel Node Biopsy This information explains your lymphatic mapping with sentinel node biopsy procedure at Memorial Sloan Kettering (MSK). You might have this procedure if you have breast cancer or melanoma. It will help your doctor see if cancer cells have spread to your lymph nodes. About Your Lymphatic System Your lymphatic system has 2 main jobs: It helps fight infection. It helps drain fluid from areas of your body. Your lymphatic system is made up of lymph nodes, lymphatic vessels, and lymphatic fluid (see Figures 1 and 2). Lymph nodes are small bean-shaped structures located along your lymphatic vessels. They filter your lymphatic fluid, taking out bacteria, viruses, cancer cells, and other waste products. A sentinel lymph node (often called a sentinel node) is the first lymph node that cancer cells might spread to. Lymphatic vessels are tiny tubes (like blood vessels) that carry lymphatic fluid to and from your lymph nodes. Lymphatic fluid is the clear fluid that travels through your lymphatic system. It carries cells that help fight infections and other diseases. Lymphatic Mapping with Sentinel Node Biopsy 1/6 Figure 1. Your lymphatic system in your breast Lymphatic Mapping with Sentinel Node Biopsy 2/6 Figure 2. Your lymphatic system in other areas of your body Lymphatic Mapping Lymphatic mapping is the first step in your sentinel node biopsy. It’s done to find the sentinel node. Lymphatic mapping can be done the day before or the day of your sentinel node biopsy. Check your appointment reminder for where to go for your lymphatic mapping procedure. -

New Developments in Imaging for Sentinel Lymph Node Biopsy in Early-Stage Oral Cavity Squamous Cell Carcinoma
cancers Review New Developments in Imaging for Sentinel Lymph Node Biopsy in Early-Stage Oral Cavity Squamous Cell Carcinoma 1 2, 3, 2 Rutger Mahieu , Josanne S. de Maar y , Eliane R. Nieuwenhuis y, Roel Deckers , Chrit Moonen 2, Lejla Alic 3 , Bennie ten Haken 3, Bart de Keizer 4 and Remco de Bree 1,* 1 Department of Head and Neck Surgical Oncology, University Medical Center Utrecht, University of Utrecht, 3584 CX Utrecht, The Netherlands; [email protected] 2 Division of Imaging and Oncology, University Medical Center Utrecht, University of Utrecht, 3584 CX Utrecht, The Netherlands; [email protected] (J.S.d.M.); [email protected] (R.D.); [email protected] (C.M.) 3 Department of Magnetic Detection & Imaging, University of Twente, 7522 NB Enschede, The Netherlands; [email protected] (E.R.N.); [email protected] (L.A.); [email protected] (B.t.H.) 4 Department of Radiology and Nuclear Medicine, University Medical Center Utrecht, 3584 CX Utrecht, The Netherlands; [email protected] * Correspondence: [email protected]; Tel.: +31-88-7550819 These authors contributed equally to this work. y Received: 11 September 2020; Accepted: 15 October 2020; Published: 20 October 2020 Simple Summary: In early-stage (cT1-2N0) oral cancer, occult lymph node metastases are present in 20–30% of patients. Accordingly, accurate staging of the clinically negative cervical nodal basin is warranted in these patients. Sentinel lymph node biopsy has proven to reliably stage the clinically negative cervical nodal basin in early-stage oral cancer. However, due to the limited resolution of conventional sentinel lymph node imaging, occult lymph node metastasis may be missed in particular circumstances. -

(CCSR) for ICD-10-PCS PROCEDURES, V2021.1
USER GUIDE: CLINICAL CLASSIFICATIONS SOFTWARE REFINED (CCSR) FOR ICD-10-PCS PROCEDURES, v2021.1 Issued December 2020 Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP) Phone: (866) 290-HCUP (4287) Email: [email protected] Website: www.hcup-us.ahrq.gov TABLE OF CONTENTS What’s New in v2021.1 of the Clinical Classifications Software Refined (CCSR) for ICD-10-PCS Procedures? .............................................................................................................................. 1 Introduction ................................................................................................................................ 2 Comparison of the CCSR for ICD-10-PCS, the Beta Versions of the CCS for ICD-10-PCS, and the CCS for ICD-9-CM ............................................................................................................... 3 Description of the CCSR for ICD-10-PCS .................................................................................. 4 Understanding the Taxonomy of the ICD-10-PCS Procedure Codes ...................................... 4 The Structure of the CCSR for ICD-10-PCS ........................................................................... 7 General Assignment Guidelines .........................................................................................11 Using the CCSR to Trend ICD-10-PCS Across Data Years .......................................................12 Using the Downloadable CCSR Files ........................................................................................12