Document Downloaded From
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2,4-Dichlorophenoxyacetic Acid
2,4-Dichlorophenoxyacetic acid 2,4-Dichlorophenoxyacetic acid IUPAC (2,4-dichlorophenoxy)acetic acid name 2,4-D Other hedonal names trinoxol Identifiers CAS [94-75-7] number SMILES OC(COC1=CC=C(Cl)C=C1Cl)=O ChemSpider 1441 ID Properties Molecular C H Cl O formula 8 6 2 3 Molar mass 221.04 g mol−1 Appearance white to yellow powder Melting point 140.5 °C (413.5 K) Boiling 160 °C (0.4 mm Hg) point Solubility in 900 mg/L (25 °C) water Related compounds Related 2,4,5-T, Dichlorprop compounds Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) 2,4-Dichlorophenoxyacetic acid (2,4-D) is a common systemic herbicide used in the control of broadleaf weeds. It is the most widely used herbicide in the world, and the third most commonly used in North America.[1] 2,4-D is also an important synthetic auxin, often used in laboratories for plant research and as a supplement in plant cell culture media such as MS medium. History 2,4-D was developed during World War II by a British team at Rothamsted Experimental Station, under the leadership of Judah Hirsch Quastel, aiming to increase crop yields for a nation at war.[citation needed] When it was commercially released in 1946, it became the first successful selective herbicide and allowed for greatly enhanced weed control in wheat, maize (corn), rice, and similar cereal grass crop, because it only kills dicots, leaving behind monocots. Mechanism of herbicide action 2,4-D is a synthetic auxin, which is a class of plant growth regulators. -

Herbicide Mode of Action Table High Resistance Risk
Herbicide Mode of Action Table High resistance risk Chemical family Active constituent (first registered trade name) GROUP 1 Inhibition of acetyl co-enzyme A carboxylase (ACC’ase inhibitors) clodinafop (Topik®), cyhalofop (Agixa®*, Barnstorm®), diclofop (Cheetah® Gold* Decision®*, Hoegrass®), Aryloxyphenoxy- fenoxaprop (Cheetah®, Gold*, Wildcat®), fluazifop propionates (FOPs) (Fusilade®), haloxyfop (Verdict®), propaquizafop (Shogun®), quizalofop (Targa®) Cyclohexanediones (DIMs) butroxydim (Factor®*), clethodim (Select®), profoxydim (Aura®), sethoxydim (Cheetah® Gold*, Decision®*), tralkoxydim (Achieve®) Phenylpyrazoles (DENs) pinoxaden (Axial®) GROUP 2 Inhibition of acetolactate synthase (ALS inhibitors), acetohydroxyacid synthase (AHAS) Imidazolinones (IMIs) imazamox (Intervix®*, Raptor®), imazapic (Bobcat I-Maxx®*, Flame®, Midas®*, OnDuty®*), imazapyr (Arsenal Xpress®*, Intervix®*, Lightning®*, Midas®* OnDuty®*), imazethapyr (Lightning®*, Spinnaker®) Pyrimidinyl–thio- bispyribac (Nominee®), pyrithiobac (Staple®) benzoates Sulfonylureas (SUs) azimsulfuron (Gulliver®), bensulfuron (Londax®), chlorsulfuron (Glean®), ethoxysulfuron (Hero®), foramsulfuron (Tribute®), halosulfuron (Sempra®), iodosulfuron (Hussar®), mesosulfuron (Atlantis®), metsulfuron (Ally®, Harmony®* M, Stinger®*, Trounce®*, Ultimate Brushweed®* Herbicide), prosulfuron (Casper®*), rimsulfuron (Titus®), sulfometuron (Oust®, Eucmix Pre Plant®*, Trimac Plus®*), sulfosulfuron (Monza®), thifensulfuron (Harmony®* M), triasulfuron (Logran®, Logran® B-Power®*), tribenuron (Express®), -

Exposure to Herbicides in House Dust and Risk of Childhood Acute Lymphoblastic Leukemia
Journal of Exposure Science and Environmental Epidemiology (2013) 23, 363–370 & 2013 Nature America, Inc. All rights reserved 1559-0631/13 www.nature.com/jes ORIGINAL ARTICLE Exposure to herbicides in house dust and risk of childhood acute lymphoblastic leukemia Catherine Metayer1, Joanne S. Colt2, Patricia A. Buffler1, Helen D. Reed3, Steve Selvin1, Vonda Crouse4 and Mary H. Ward2 We examine the association between exposure to herbicides and childhood acute lymphoblastic leukemia (ALL). Dust samples were collected from homes of 269 ALL cases and 333 healthy controls (o8 years of age at diagnosis/reference date and residing in same home since diagnosis/reference date) in California, using a high-volume surface sampler or household vacuum bags. Amounts of agricultural or professional herbicides (alachlor, metolachlor, bromoxynil, bromoxynil octanoate, pebulate, butylate, prometryn, simazine, ethalfluralin, and pendimethalin) and residential herbicides (cyanazine, trifluralin, 2-methyl-4- chlorophenoxyacetic acid (MCPA), mecoprop, 2,4-dichlorophenoxyacetic acid (2,4-D), chlorthal, and dicamba) were measured. Odds ratios (OR) and 95% confidence intervals (CI) were estimated by logistic regression. Models included the herbicide of interest, age, sex, race/ethnicity, household income, year and season of dust sampling, neighborhood type, and residence type. The risk of childhood ALL was associated with dust levels of chlorthal; compared to homes with no detections, ORs for the first, second, and third tertiles were 1.49 (95% CI: 0.82–2.72), 1.49 (95% CI: 0.83–2.67), and 1.57 (95% CI: 0.90–2.73), respectively (P-value for linear trend ¼ 0.05). The magnitude of this association appeared to be higher in the presence of alachlor. -
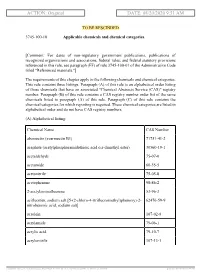
ACTION: Original DATE: 08/20/2020 9:51 AM
ACTION: Original DATE: 08/20/2020 9:51 AM TO BE RESCINDED 3745-100-10 Applicable chemicals and chemical categories. [Comment: For dates of non-regulatory government publications, publications of recognized organizations and associations, federal rules, and federal statutory provisions referenced in this rule, see paragraph (FF) of rule 3745-100-01 of the Administrative Code titled "Referenced materials."] The requirements of this chapter apply to the following chemicals and chemical categories. This rule contains three listings. Paragraph (A) of this rule is an alphabetical order listing of those chemicals that have an associated "Chemical Abstracts Service (CAS)" registry number. Paragraph (B) of this rule contains a CAS registry number order list of the same chemicals listed in paragraph (A) of this rule. Paragraph (C) of this rule contains the chemical categories for which reporting is required. These chemical categories are listed in alphabetical order and do not have CAS registry numbers. (A) Alphabetical listing: Chemical Name CAS Number abamectin (avermectin B1) 71751-41-2 acephate (acetylphosphoramidothioic acid o,s-dimethyl ester) 30560-19-1 acetaldehyde 75-07-0 acetamide 60-35-5 acetonitrile 75-05-8 acetophenone 98-86-2 2-acetylaminofluorene 53-96-3 acifluorfen, sodium salt [5-(2-chloro-4-(trifluoromethyl)phenoxy)-2- 62476-59-9 nitrobenzoic acid, sodium salt] acrolein 107-02-8 acrylamide 79-06-1 acrylic acid 79-10-7 acrylonitrile 107-13-1 [ stylesheet: rule.xsl 2.14, authoring tool: RAS XMetaL R2_0F1, (dv: 0, p: 185720, pa: -

AP-42, CH 9.2.2: Pesticide Application
9.2.2PesticideApplication 9.2.2.1General1-2 Pesticidesaresubstancesormixturesusedtocontrolplantandanimallifeforthepurposesof increasingandimprovingagriculturalproduction,protectingpublichealthfrompest-bornediseaseand discomfort,reducingpropertydamagecausedbypests,andimprovingtheaestheticqualityofoutdoor orindoorsurroundings.Pesticidesareusedwidelyinagriculture,byhomeowners,byindustry,andby governmentagencies.Thelargestusageofchemicalswithpesticidalactivity,byweightof"active ingredient"(AI),isinagriculture.Agriculturalpesticidesareusedforcost-effectivecontrolofweeds, insects,mites,fungi,nematodes,andotherthreatstotheyield,quality,orsafetyoffood.Theannual U.S.usageofpesticideAIs(i.e.,insecticides,herbicides,andfungicides)isover800millionpounds. AiremissionsfrompesticideusearisebecauseofthevolatilenatureofmanyAIs,solvents, andotheradditivesusedinformulations,andofthedustynatureofsomeformulations.Mostmodern pesticidesareorganiccompounds.EmissionscanresultdirectlyduringapplicationorastheAIor solventvolatilizesovertimefromsoilandvegetation.Thisdiscussionwillfocusonemissionfactors forvolatilization.Thereareinsufficientdataavailableonparticulateemissionstopermitemission factordevelopment. 9.2.2.2ProcessDescription3-6 ApplicationMethods- Pesticideapplicationmethodsvaryaccordingtothetargetpestandtothecroporothervalue tobeprotected.Insomecases,thepesticideisapplieddirectlytothepest,andinotherstothehost plant.Instillothers,itisusedonthesoilorinanenclosedairspace.Pesticidemanufacturershave developedvariousformulationsofAIstomeetboththepestcontrolneedsandthepreferred -

Cascade Range
United States bepartment of Agriculture Herbicide Forest Service Pacific Northwest Forest and Range and Conifer Experiment Station Research Paper PNW-292 Options for Reforesting October, 1981 Upper Slopes in the Cascade Range Edward J. Dimock II ROCKY Mof 'NTATN STATION This publication reports research involving pesticides. It does not contain recommendations for their use, nor does it imply that the uses discussed have been registered. All uses of pesticides must be registered by appropriate State and/or Federal agencies before they can be recommended. CAUTION: Pesticides can be injurious to humans, domestic animals, desirable Author plants, and fish or other wildlife -- if they I~ m w ~llmml are not handled or applied properly. Use EDWARD J. DIMOCK II is principal all pesticides selectively and carefully. silviculturist, Forestry Sciences Follow recommended practices for the Laboratory, 3200 Jefferson Way, disposal of surplus pesticides and Corvallis, Oregon 97331. pesticide containers. Abstract Summary Dimock, Edward J. I1. Herbicide and Nine herbicides (asulam, atrazine, Similar survival increases were also conifer options for reforesting upper bromacil, cyanazine, dalapon, consistently achieved with atrazine + slopes in the Cascade Range. Res. glyphosate, hexazinone, pronamide, and dalapon mixtures. Most successful Pap. PNW-292. Portland, OR: U.S. terbacil) were evaluated for control of applications of mixture produced 3-year Department of Agriculture, Forest sedge (Carex spp.) and beargrass survival of 62, 77, and 14 percent for white Service, Pacific Northwest Forest (Xerophyllum tenax [Pursh] Nutt.) to aid pine at the same three localities above. and Range Experiment Station; establishment of newly planted seedlings Survival of other conifers resulting from 1981.14 p. -

Weed Control Guide for Ohio, Indiana and Illinois
Pub# WS16 / Bulletin 789 / IL15 OHIO STATE UNIVERSITY EXTENSION Tables Table 1. Weed Response to “Burndown” Herbicides .............................................................................................19 Table 2. Application Intervals for Early Preplant Herbicides ............................................................................... 20 Table 3. Weed Response to Preplant/Preemergence Herbicides in Corn—Grasses ....................................30 WEED Table 4. Weed Response to Preplant/Preemergence Herbicides in Corn—Broadleaf Weeds ....................31 Table 5. Weed Response to Postemergence Herbicides in Corn—Grasses ...................................................32 Table 6. Weed Response to Postemergence Herbicides in Corn—Broadleaf Weeds ..................................33 2015 CONTROL Table 7. Grazing and Forage (Silage, Hay, etc.) Intervals for Herbicide-Treated Corn ................................. 66 OHIO, INDIANA Table 8. Rainfast Intervals, Spray Additives, and Maximum Crop Size for Postemergence Corn Herbicides .........................................................................................................................................................68 AND ILLINOIS Table 9. Herbicides Labeled for Use on Field Corn, Seed Corn, Popcorn, and Sweet Corn ..................... 69 GUIDE Table 10. Herbicide and Soil Insecticide Use Precautions ......................................................................................71 Table 11. Weed Response to Herbicides in Popcorn and Sweet Corn—Grasses -

List of Herbicide Groups
List of herbicides Group Scientific name Trade name clodinafop (Topik®), cyhalofop (Barnstorm®), diclofop (Cheetah® Gold*, Decision®*, Hoegrass®), fenoxaprop (Cheetah® Gold* , Wildcat®), A Aryloxyphenoxypropionates fluazifop (Fusilade®, Fusion®*), haloxyfop (Verdict®), propaquizafop (Shogun®), quizalofop (Targa®) butroxydim (Falcon®, Fusion®*), clethodim (Select®), profoxydim A Cyclohexanediones (Aura®), sethoxydim (Cheetah® Gold*, Decision®*), tralkoxydim (Achieve®) A Phenylpyrazoles pinoxaden (Axial®) azimsulfuron (Gulliver®), bensulfuron (Londax®), chlorsulfuron (Glean®), ethoxysulfuron (Hero®), foramsulfuron (Tribute®), halosulfuron (Sempra®), iodosulfuron (Hussar®), mesosulfuron (Atlantis®), metsulfuron (Ally®, Harmony®* M, Stinger®*, Trounce®*, B Sulfonylureas Ultimate Brushweed®* Herbicide), prosulfuron (Casper®*), rimsulfuron (Titus®), sulfometuron (Oust®, Eucmix Pre Plant®*), sulfosulfuron (Monza®), thifensulfuron (Harmony®* M), triasulfuron, (Logran®, Logran® B Power®*), tribenuron (Express®), trifloxysulfuron (Envoke®, Krismat®*) florasulam (Paradigm®*, Vortex®*, X-Pand®*), flumetsulam B Triazolopyrimidines (Broadstrike®), metosulam (Eclipse®), pyroxsulam (Crusader®Rexade®*) imazamox (Intervix®*, Raptor®,), imazapic (Bobcat I-Maxx®*, Flame®, Midas®*, OnDuty®*), imazapyr (Arsenal Xpress®*, Intervix®*, B Imidazolinones Lightning®*, Midas®*, OnDuty®*), imazethapyr (Lightning®*, Spinnaker®) B Pyrimidinylthiobenzoates bispyribac (Nominee®), pyrithiobac (Staple®) C Amides: propanil (Stam®) C Benzothiadiazinones: bentazone (Basagran®, -

Pesticides EPA 738-R-06-008 Environmental Protection and Toxic Substances April 2006 Agency (7508P)
Simazine RED April 6, 2006 United States Prevention, Pesticides EPA 738-R-06-008 Environmental Protection and Toxic Substances April 2006 Agency (7508P) Reregistration Eligibility Decision for Simazine Page 1 of 266 Reregistration Eligibility Decision (RED) Document for Simazine List A Case Number 0070 Approved by: Date: April 6, 2006 Debra Edwards, Ph. D. Director Special Review and Reregistration Division Page 2 of 266 Table of Contents Simazine Reregistration Eligibility Decision Team ................................................................... 5 Glossary of Terms and Abbreviations ........................................................................................ 6 Abstract.......................................................................................................................................... 8 I. Introduction............................................................................................................................... 9 II. Chemical Overview................................................................................................................ 10 A. Chemical Identity ................................................................................................................10 B. Use and Usage Profile .........................................................................................................11 C. Tolerances............................................................................................................................12 III. Summary of Risk Assessments -

Cyanazine in Drinking-Water
WHO/SDE/WSH/03.04/60 English only Cyanazine in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality ______________________ Originally published in Guidelines for drinking-water quality, 2nd ed. Addendum to Vol. 2. Health criteria and other supporting information. World Health Organization, Geneva, 1998. © World Health Organization 2003 All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use. -

5. Key Post-Emergent Modes of Action
5. KEY POST-EMERGENT MODES OF ACTION When new mode of action herbicides are first introduced, of this enzyme is reduction in the production of fatty acids manufacturers typically provide robust formulations and use required for construction of cell membranes needed for rates. There is often a high level of “forgiveness” in the label. new cell production. As resistant populations are selected over time, there is The ACCase enzyme in most broadleaf plants is insensitive sometimes a period where the herbicide may still be useful, to herbicides from this herbicide mode of action, and hence albeit with reduced performance. In these situations of low- there is acceptable crop tolerance in most broadleaf crops level or emerging resistance, it is critical that users seek to and no efficacy on most broadleaf weeds. Some exceptions maximise application conditions to ensure everything possible exist. For example, haloxyfop is able to control the broadleaf is done to enhance the herbicide performance. weed storksbill or geranium (Erodium spp.) while high rates of clethodim can damage canola, particularly when flowering. Understanding how each of the key modes of action available for post-emergent weed control work, how they The three sub-groups of Group A herbicides bind to the target enter and translocate in the plant, and what is required to enzyme at slightly different, and overlapping, amino acids. This differential binding can lead to differences in target site maximise efficacy is critical knowledge for maximising field herbicide resistance patterns both between and within the performance. sub groups (refer to the Acetyl CoA Carboxylase inhibitors This chapter coves the key modes of actions used for post- section under section 6.3.1.1. -

Occurrence of Cotton Pesticides in Surface Water of the Mississippi Embayment
Occurrence of Cotton Pesticides in Surface Water of the Mississippi Embayment £ Michael Thurman, Lisa R. Zimmerman, Elisabeth A. Scribner, and Richard H. Coupe Jr. This fact sheet introduces a study of the fluometuron is a cotton herbicide used The three most-used pesticides are nonpoint-source occurrence of pesticides primarily in Mississippi and in the herbicides (monosodium methylarsonate, in water of the cotton-growing areas of eastern coastal plain (fig. 2; Battaglin and trifluralin, and fluometuron). the Southern United States with special Goolsby, 1995) but is little used in other emphasis on the Mississippi Embayment cotton-growing areas. Study Area (fig. 1). This study is being conducted by the U.S. Geological Survey (USGS) The geographic usage information A major area for cotton production in Organic Geochemistry Research Group, displayed in figure 2 can be useful for the United States is the Mississippi which investigates the fate and transport designing regional water-quality surveys Embayment (figs. 1 and 4). The area of agricultural chemicals in the and for methods-development strategies consists of parts of Arkansas, Kentucky, environment. The purpose of this fact for a compound such as fluometuron. Louisiana, Mississippi, Missouri, and sheet is to give an overview of the cotton- Another method to visualize cotton Tennessee. Rainfall amounts of as much growing areas of the United States, to pesticide usage in the United States is the as 60 inches per year and relatively flat, provide usage data for cotton pesticides bar graph shown in figure 3. The com only slightly permeable soils in the United States, to present pounds shown account for nearly all of characterized by large runoff volumes and information on the types of pesticide the pesticides used in cotton-growing low relief are typical of this area.