Response of Chilo Partellus (Swinhoe)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Downloaded from the Same Database
G C A T T A C G G C A T genes Article Genetic Basis of Maize Resistance to Multiple Insect Pests: Integrated Genome-Wide Comparative Mapping and Candidate Gene Prioritization A. Badji 1,* , D. B. Kwemoi 2, L. Machida 3 , D. Okii 1, N. Mwila 1, S. Agbahoungba 4, F. Kumi 5 , A. Ibanda 1 , A. Bararyenya 1, M. Solemanegy 1, T. Odong 1, P. Wasswa 1, M. Otim 2, G. Asea 2, M. Ochwo-Ssemakula 1, H. Talwana 1, S. Kyamanywa 1 and P. Rubaihayo 1 1 Department of Agricultural Production, Makerere Univesity, P.O. Box 7062 Kampala, Uganda; [email protected] (D.O.); [email protected] (N.M.); [email protected] (A.I.); [email protected] (A.B.); [email protected] (M.S.); [email protected] (T.O.); [email protected] (P.W.); [email protected] (M.O.-S.); [email protected] (H.T.); [email protected] (S.K.); [email protected] (P.R.) 2 Cereals Program, National Crop Resource Research Institute, P.O. Box 7084 Kampala, Uganda; [email protected] (D.B.K.); [email protected] (M.O.); [email protected] (G.A.) 3 Alliance Bioversity International-CIAT, P.O. Box 24384 Kampala, Uganda; [email protected] 4 Laboratory of Applied Ecology, University of Abomey-Calavi, 01BP 526 Cotonou, Benin; [email protected] 5 Department of Crop Science, University of Cape Coast, P.O. Box 5007 PMB Cape Coast, Ghana; [email protected] * Correspondence: [email protected] Received: 19 May 2020; Accepted: 1 June 2020; Published: 24 June 2020 Abstract: Several species of herbivores feed on maize in field and storage setups, making the development of multiple insect resistance a critical breeding target. -

Response of Chilo Partellus (Lepidoptera: Crambidae) to Bt Maize in South Africa
Response of Chilo partellus (Lepidoptera: Crambidae) to Bt maize in South Africa J Vorster orcid.org/0000-0001-8126-6860 Dissertation submitted in fulfilment of the requirements for the Masters degree in Environmental Science at the North-West University Supervisor: Prof J van den Berg Co-supervisor: Prof MJ du Plessis Assistant supervisor: Dr A Erasmus Graduation May 2018 23441674 ACKNOWLEDGEMENTS This dissertation would not have been possible without the help of so many people. I am blessed and very grateful to have them in my life. I would like to start with our God Almighty and our Saviour who bestowed upon me the strength, wisdom and peace of mind to finish this project and who also have sent me these blessed people in my life. I would like to thank Prof. Johnnie van den Berg and Dr. Annemie Erasmus for all the guidance and support they have given me. You taught me that small things can make a big difference. Statistics can be difficult sometimes and I thank Prof. Hannalene du Plessis and Prof. Suria Elis for the help with the statistics. Thank you to all the staff at the ARC-GCI that assisted me with the trials in the lab and the planting. Elrine Strydom, Mabel du Toit, Heidi Meyer and Ursula du Plessis, thank you for the countless after hours we had to spend and for the warm hearted kindness you have given me. I would also like to thank my parents whom I dearly love for all the encouragement and motivation to do my best. You taught me that hard work does not come easily, but the fruit that you pick from it is what motivates us. -
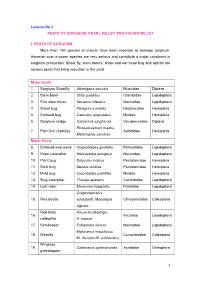
Lecture No 3 PESTS of SORGHUM, PEARL MILLET and FINGER MILLET
Lecture No 3 PESTS OF SORGHUM, PEARL MILLET AND FINGER MILLET I. PESTS OF SORGHUM More than 150 species of insects have been reported to damage sorghum. However over a dozen species are very serious and constitute a major constraint in sorghum production. Shoot fly, stem borers, shoot and ear head bug and aphids are serious pests that bring reduction in the yield. Major pests 1. Sorghum Shootfly Atherigona soccata Muscidae Diptera 2. Stem borer Chilo partellus Crambidae Lepidoptera 3. Pink stem borer Sesamia inferens Noctuidae Lepidoptera 4 Shoot bug Peregrinus maidis Delphacidae Hemiptera 5. Earhead bug Calocoris angustatus Miridae Hemiptera 6. Sorghum midge Contarinia sorghicola Cecidomyiidae Diptera Rhopalosiphum maidis, 7. Plant lice (Aphids) Aphididae Hemiptera Melanaphis sacchari Minor Pests 8. Earhead web worm Cryptoblabes gnidiella Pyraustidae Lepidoptera 9. Gram caterpillar Helicoverpa armigera Noctuidae Lepidoptera 10. Plant bug Dolycoris indicus Pentatomidae Hemiptera 11. Stink bug Nezara viridula Pentatomidae Hemiptera 12. Mirid bug Creontiades pallidifer Miridae Hemiptera 13. Slug caterpillar Thosea apierens Cochlididae Lepidoptera 14. Leaf roller Marasmia trapezalis Pyralidae Lepidoptera Cryptocephalus 15. Flea beetle schestedii, Monolepta Chrysomelidae Coleoptera signata Red hairy Amsacta albistriga, 16. Arctiidae Lepidoptera caterpillar A. moorei 17. Semilooper Eublemma silicula Noctuidae Lepidoptera Myllocerus maculosus 18. Weevils Curculionidae Coleoptera M. discolor,M. subfaciatus Wingless 19. Colemania sphenaroides Acrididae Orthoptera grasshopper MAJOR PESTS 1.Sorghum Shootfly: Atherigona soccata (Muscidae: Diptera) Distribution and status Maharashtra, Andhra Pradesh, Tamil Nadu and Karnataka Host range: Maize, ragi, bajra, rice, wheat and grasses Damage symptoms The maggot on hatching migrates to the upper surface of leaf and enters between the leaf sheath and stem. -

Crambidae Biosecurity Occurrence Background Subfamilies Short Description Diagnosis
Diaphania nitidalis Chilo infuscatellus Crambidae Webworms, Grass Moths, Shoot Borers Biosecurity BIOSECURITY ALERT This Family is of Biosecurity Concern Occurrence This family occurs in Australia. Background The Crambidae is a large, diverse and ubiquitous family of moths that currently comprises 11,500 species globally, with at least half that number again undescribed. The Crambidae and the Pyralidae constitute the superfamily Pyraloidea. Crambid larvae are concealed feeders with a great diversity in feeding habits, shelter building and hosts, such as: leaf rollers, shoot borers, grass borers, leaf webbers, moss feeders, root feeders that shelter in soil tunnels, and solely aquatic life habits. Many species are economically important pests in crops and stored food products. Subfamilies Until recently, the Crambidae was treated as a subfamily under the Pyralidae (snout moths or grass moths). Now they form the superfamily Pyraloidea with the Pyralidae. The Crambidae currently consists of the following 14 subfamilies: Acentropinae Crambinae Cybalomiinae Glaphyriinae Heliothelinae Lathrotelinae Linostinae Midilinae Musotiminae Odontiinae Pyraustinae Schoenobiinae Scopariinae Spilomelinae Short Description Crambid caterpillars are generally cylindrical, with a semiprognathous head and only primary setae (Fig 1). They are often plainly coloured (Fig. 16, Fig. 19), but can be patterned with longitudinal stripes and pinacula that may give them a spotted appearance (Fig. 10, Fig. 11, Fig. 14, Fig. 22). Prolegs may be reduced in borers (Fig. 16). More detailed descriptions are provided below. This factsheet presents, firstly, diagnostic features for the Pyraloidea (Pyralidae and Crambidae) and then the Crambidae. Information and diagnostic features are then provided for crambids listed as priority biosecurity threats for northern Australia. -

Assessment of Grain Yield Losses in Pearl Millet Due to the Millet Stemborer, Coniesta Ignefusalis (Hampson)
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications: Department of Entomology Entomology, Department of 2003 ASSESSMENT OF GRAIN YIELD LOSSES IN PEARL MILLET DUE TO THE MILLET STEMBORER, CONIESTA IGNEFUSALIS (HAMPSON) Aissetou Drame-Yaye Université Abdou Moumouni Ousmane Youm University of Nebraska-Lincoln, [email protected] Jonathan N. Ayertey University of Ghana Follow this and additional works at: https://digitalcommons.unl.edu/entomologyfacpub Part of the Entomology Commons Drame-Yaye, Aissetou; Youm, Ousmane; and Ayertey, Jonathan N., "ASSESSMENT OF GRAIN YIELD LOSSES IN PEARL MILLET DUE TO THE MILLET STEMBORER, CONIESTA IGNEFUSALIS (HAMPSON)" (2003). Faculty Publications: Department of Entomology. 328. https://digitalcommons.unl.edu/entomologyfacpub/328 This Article is brought to you for free and open access by the Entomology, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications: Department of Entomology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Insect Sci. Applic. Vol. 23, No. 3,Coniesta pp. 259–265, ignefusalis 2003 larval establishment and damage 0191-9040/03 $3.00 + 0.00 259 Printed in Kenya. All rights reserved © 2003 ICIPE ASSESSMENT OF GRAIN YIELD LOSSES IN PEARL MILLET DUE TO THE MILLET STEMBORER, CONIESTA IGNEFUSALIS (HAMPSON) AISSETOU DRAME-YAYE1, OUSMANE YOUM2 AND JONATHAN N. AYERTEY3 1Faculté d’Agronomie, Université Abdou Moumouni , BP 12040 Niamey, Niger; 2 ICRISAT Sahelian Centre, BP 12404, Niamey, Niger; 3 Department of Crop Science, University of Ghana, P. O. Box LG 44, Legon Accra, Ghana (Accepted 4 June 2003) Abstract—Studies were conducted at the ICRISAT Sahelian Centre, Niger, to assess damage and yield loss by the millet stemborer, Coniesta ignefusalis (Lepidoptera: Pyralidae) on Pennisetum glaucum (L.) R. -

Establishment of the Fungal Entomopathogen Beauveria Bassiana As an Endophyte in Sugarcane, Saccharum Officinarum
Fungal Ecology 35 (2018) 70e77 Contents lists available at ScienceDirect Fungal Ecology journal homepage: www.elsevier.com/locate/funeco Establishment of the fungal entomopathogen Beauveria bassiana as an endophyte in sugarcane, Saccharum officinarum * Trust Kasambala Donga a, b, Fernando E. Vega c, Ingeborg Klingen d, a Department of Plant Sciences, Norwegian University of Life Sciences (NMBU), Campus ÅS, Universitetstunet 3, 1433, Ås, Norway b Lilongwe University of Agriculture and Natural Resources (LUANAR), P.O. Box 219, Lilongwe, Malawi c Sustainable Perennial Crops Laboratory, United States Department of Agriculture (USDA), Agricultural Research Service, Beltsville, MD, 20705, USA d Division for Biotechnology and Plant Health, Norwegian Institute of Bioeconomy Research (NIBIO), Høgskoleveien 7, 1431, Ås, Norway article info abstract Article history: We investigated the ability of the fungal entomopathogen Beauveria bassiana strain GHA to endo- Received 18 April 2018 phytically colonize sugarcane (Saccharum officinarum) and its impact on plant growth. We used foliar Received in revised form spray, stem injection, and soil drench inoculation methods. All three inoculation methods resulted in 18 June 2018 B. bassiana colonizing sugarcane tissues. Extent of fungal colonization differed significantly with inoc- Accepted 28 June 2018 ulation method (c2 ¼ 20.112, d. f. ¼ 2, p < 0.001), and stem injection showed the highest colonization level followed by foliar spray and root drench. Extent of fungal colonization differed significantly with Corresponding Editor: James White Jr. plant part (c2 ¼ 33.072, d. f. ¼ 5, p < 0.001); stem injection resulted in B. bassiana colonization of the stem and to some extent leaves; foliar spray resulted in colonization of leaves and to some extent, the stem; Keywords: and soil drench resulted in colonization of roots and to some extent the stem. -

Interactions of Spotted Stem Borer Chilo Partellus with Wild Relatives of Sorghum
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by ICRISAT Open Access Repository Plant Breeding doi:10.1111/j.1439-0523.2012.01966.x Ó 2012 Blackwell Verlag GmbH Interactions of spotted stem borer Chilo partellus with wild relatives of sorghum 1,6 2 3 1 Venkateswaran KAMALA , Hari C. SHARMA , Daggu MANOHAR R AO , Kodeboyina S. VARAPRASAD , 4 5 Paula J. BRAMEL and Subhash CHANDRA 1National Bureau of Plant Genetic Resources, Regional Station, Rajendranagar, Hyderabad 500 030, Andhra Pradesh, India; 2International Crops Research Institute for the Semi-Arid Tropics, Patancheru 502 324, Andhra Pradesh, India; 3Department of Genetics, Osmania University, Hyderabad 500007, Andhra Pradesh, India; 4International Institute for Tropical Agriculture, Ibadan, Nigeria; 5Department of Primary Industries, Tatura 3616, Vic., Australia; 6Corresponding author, E-mail: [email protected] With 5 tables Received August 10, 2011/Accepted February 22, 2012 Communicated by T. Lu¨bberstedt Abstract owing to insect pests have been estimated to be over $1000 The levels of resistance to spotted stem borer (Chilo partellus)in million annually in the semi-arid tropics (ikisan.com 2000). sorghum are low to moderate. We therefore evaluated wild relatives of Global sorghum crop losses owing to stem borer damage have sorghum to identify accessions with high levels of resistance to this pest been estimated at over US$ 300 million annually (ICRISAT, and studied the mechanisms of resistance. Heterosorghum (Sorghum 1992; Sharma 1997). In general, yield losses range between 5% laxiflorum), Para-sorghum (S. australiense, S. purpureo-sericeum, and 10%, especially when the infestation occurs early. -

Physicochemical Mechanisms of Resistance in Sorghum to Chilo Partellus (Swinhoe)
Indian Journal of Experimental Biology Vol. 56, January 2018, pp. 29-38 Physicochemical mechanisms of resistance in sorghum to Chilo partellus (Swinhoe) Mukesh K Dhillon1* & DP Chaudhary2 1Division of Entomology, ICAR-Indian Agricultural Research Institute, New Delhi 110 012, India 2Biochemistry Laboratory, ICAR-Indian Institute of Maize Research, Pusa Campus, New Delhi 110 012, India Received 03 May 2017; revised 20 August 2017 Variation in nutritional components and amounts of secondary metabolites not only affects the growth, development and survival of insect herbivores but also indirectly influences expression of host plant resistance to insects. In this study, we examined the role of different biochemical and morphological factors in sorghum as host plant defense against the spotted stem borer, Chilo partellus (Swinhoe). The genotypes IS 2205 and IS 2123 suffered lower deadheart incidence, and exhibited deleterious effects on development and survival of C. partellus, followed by ICSV 700, ICSV 708 and ICSV 25066 than the susceptible check, Swarna. The anthocyanin pigmentation in sorghum seedlings and C. partellus deadhearts were found significantly and negatively correlated with the larval period (r = −0.60** to −0.88**), while positively correlated with the larval and pupal weights, and larval survival and adult emergence (r = 0.58* to 0.95**). Conversely, the numbers of C. partellus exit holes in the stalk, larvae recovered, number of tunnels and stem tunneling length were significantly and positively correlated (*, ** = P ≤0.05 and 0.01, respectively) with the larval period (r = 0.72** to 0.89**), but significantly and negatively correlated with larval and pupal weights, larval survival and adult emergence (r = −0.54* to −0.84**). -

Life Cycle of Chilo Partellus (Swinhoe) (Lepidoptera: Pyralidae) on an Artificial Diets
Received: 04th July-2013 Revised: 09th July-2013 Accepted: 13th July-2013 Research article LIFE CYCLE OF CHILO PARTELLUS (SWINHOE) (LEPIDOPTERA: PYRALIDAE) ON AN ARTIFICIAL DIETS Balaji M. Panchal* and Manvendra S. Kachole Department of Biochemistry, Dr. Babasaheb Ambedkar Marathawada University Aurangabad, Maharashtra 431004, India. *Corresponding author: [email protected] Mobile no: +919689707466 Fax no: +91-240-2375275 ABSTRACT: Chilo partellus (Swinhoe) (Lepidoptera: Pyralidae) are major insect pests of Sorghum bicolor (L) Moench, the main staple food crop in India. Life cycle of C. partellus (Swinhoe) (Lepidoptera: Pyralidae) was studied under laboratory conditions by using artificial diet. The research revealed that the C. partellus (Swinhoe) (Lepidoptera: Pyralidae) was completed its life cycle in 30-40 days. Larval growth and development was significantly faster on an artificial diet. Feeding by fourth-instar C. partellus larvae was significantly higher than third- instar larvae. Feeding on an artificial diet the C. partellus pupal period was 7-10 days. Eggs hatch in five to six days, egg hatch from 45-78% in the first generation. Key Words: Chilo partellus larvae, artificial diet, advanced rearing techniques. INTRODUCTION Insect pests are the major constraints on its production and productivity, of which stem borers mainly C. partellus (Swinhoe) (Lepidoptera: Pyralidae) are most important worldwide [1]. C. partellus (Swinhoe) (Lepidoptera: Pyralidae) are the predominant stem borer species in lowland areas of the region [2]. It is becoming increasingly important at higher elevations [3]. The stem borer, C. partellus (Swinhoe) is a serious pest of cereals; Sorghum bicolor (L) Moench and Zea mays in various parts of Asia and Africa [4-6]. -

Chilo Partellus
CAPS Pest Datasheets provide pest-specific information to support planning and completing early detection surveys. Chilo partellus Scientific Name Chilo partellus (Swinhoe, 1886) Synonyms: Chilo zonellus (Swinhoe, 1884) Crambus zonellus Swinhoe, 1885 Crambus partellus Swinhoe, 1885 Argyria lutulentalis Tams, 1932 Chilo lutulentalis Tams, 1932 Common Name Spotted stem borer, spotted stemborer, Figure 1. Adult female Chilo partellus moth. maize and jowar borer, maize stem borer, Photo by Paul-Andre Calatayud, IRD-ICIPE, Nairobi. Used with permission. durra stalk borer, spotted stalk borer, spotted sorghum stem borer, pink borer Type of Pest Moth, stem borer Taxonomic Position Class: Insecta, Order: Lepidoptera, Family: Crambidae Reason for Inclusion in Manual Priority pest for Corn Commodity Survey, 2017 - present Pest Description Eggs: Eggs are flat, scale-like, and cream colored (Whittle and Ferguson, 1988). They are laid in clusters of 10 to 80 (Harris, 1990) and darken in color as they develop (Fig. 2). Larvae: Caterpillars are cream-colored with four longitudinal stripes (Meijerman and Ulenberg, 1998) (Fig. 3). Diapausing larvae may not have Figure 2. Stages of egg development: i. stripes. Larvae have obvious dark brown spots newly laid. ii. brownheads. iii. blackheads. on their backs (Meijerman and Ulenberg, 1998). Photo by Dr. K. V. Seshu Reddy. Used The head capsule, prothoracic shield, and anal with permission. Version 1 June 14, 2019 1 shield are brown. The crochets1 on the prolegs2 form a circle with three alternating lengths in some parts. Spiracles are black and oval-shaped (Meijerman and Ulenberg, 1998). Pupae: The edges of abdominal segments 5, 6, and 7 have rough patches (Whittle and Ferguson, 1988) and the last segment of the abdomen has eight to nine prominent points (Whittle and Ferguson, 1988). -

Effects of Life History, Domestication, and Breeding of Zea on the Specialist Herbivore Dalbulus Maidis
EFFECTS OF LIFE HISTORY, DOMESTICATION, AND BREEDING OF ZEA ON THE SPECIALIST HERBIVORE DALBULUS MAIDIS (HEMIPTERA: CICADELLIDAE) A Thesis by EDWIN BELLOTA VILLAFUERTE Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Co-Chairs of Committee, Julio Bernal Raul F. Medina Committee Members, Micky D. Eubanks Michael V. Kolomiets Head of Department, David Ragsdale May 2013 Major Subject: Entomology Copyright 2013 Edwin Bellota Villafuerte ABSTRACT A suite of plants from the maize genus Zea L. (Poaceae) and the specialist herbivore Dalbulus maidis (DeLong and Wolcott, 1923) (Hemiptera: Cicadellidae) were used to test the hypotheses that anti-herbivore defenses are affected by plant life-history evolution and human intervention through domestication and breeding for high yield. The suite of plants included a commercial hybrid maize (Zea mays ssp. mays L.), a landrace maize, two populations of annual Balsas teosinte (Z. mays ssp. parviglumis Iltis & Doebley), and perennial teosinte (Z. diploperennis Iltis, Doebley & Guzman). Leaf toughness and pubescence, oviposition preference, and feeding and oviposition acceptance parameters were compared among the suite of host plants looking for effects of transitions in life history (perennial to annual teosinte), domestication (annual teosinte to landrace maize), and breeding (landrace maize to hybrid maize) on defenses against D. maidis. Observations on leaf toughness suggested that the life history and domestication transitions weakened the plant’s resistance to penetration by the herbivore’s mouthparts and ovipositor, as expected, while observations on pubescence suggested that the breeding transition led to stronger defense in hybrid maize compared to landrace maize, contrary to expectation. -

Complex Responses of Global Insect Pests to Climate Change
bioRxiv preprint doi: https://doi.org/10.1101/425488; this version posted September 24, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Complex responses of global insect pests to climate change 2 3 Philipp Lehmann1,2,4*, Tea Ammunét3†, Madeleine Barton4†, Andrea Battisti5†, Sanford 4 D. Eigenbrode6†, Jane Uhd Jepsen7†, Gregor Kalinkat8†, Seppo Neuvonen9†, Pekka 5 Niemelä10†, Bjørn Økland11†, John S. Terblanche4†, Christer Björkman3 6 7 1Department of Zoology, Stockholm University, Sweden. 2Centre of Excellence in 8 Biological Interactions Research, Department of Biological and Environmental Science, 9 University of Jyväskylä, Finland. 3Department of Ecology, Swedish University of 10 Agricultural Sciences, Sweden. 4Centre for Invasion Biology, Department of 11 Conservation Ecology and Entomology, Stellenbosch University, South Africa. 12 5Department of Agronomy, Food, Natural Resources, Animals and the Environment, 13 University of Padova, Italy. 6Department of Plant, Soil and Entomological Sciences, 14 University of Idaho, United States of America. 7Department of Arctic Ecology, 15 Norwegian Institute for Nature Research, Norway. 8Department of Biology and Ecology 16 of Fishes, Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Germany. 17 9Natural Resources Institute Finland, Finland. 10Biodiversity Unit, University of Turku, 18 Finland.11Norwegian Institute of Bioeconomy Research, Norway. 19 20 *Corresponding author: Philipp Lehmann, [email protected] 21 †Contributing authors listed alphabetically. 1 bioRxiv preprint doi: https://doi.org/10.1101/425488; this version posted September 24, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder.