Complex Responses of Global Insect Pests to Climate Change
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
The Seasonal Abundance and Impact of Predatory Arthropods on Helicoverpa Species in Australian Cotton Fields
The Seasonal Abundance and Impact of Predatory Arthropods on Helicoverpa Species in Australian Cotton Fields by John Newton Stanley B.Rur. Sc. (University of New England) A thesis submitted for the degree of Doctor of Philosophy from the University of New England Department of Agronomy and Soil Science September 1997 Many Thanks to the Following: Associate Professor Peter Gregg, who, as sole supervisor, provided a broad field of opportunities, as well as cotton, for the discovery of entomological things. Thank you for your guidance and support. Mr. Richard Browne, of Auscott Pty. Ltd., and Ben and Dave Coulton, of Coulton Farming Ltd. for allowing me access to their crops and for adjusting their cultural practices to accommodate experimental designs. Particular thanks to Terry Haynes (Senior Agronomist; Auscott Pty. Ltd. Moree) for discussions on pest management and generously supplying assistance at critical times. The taxonomists who identified material for the insect survey. Mr. L. Bauer (Thysanoptera) Dr. A. Calder (Coleoptera) Dr. M. Carver (Homoptera and Trichogrammatidae) Dr. D. H. Colless (Diptera) Dr. M. J. Fletcher (Cicadellidae) Dr. M. R. Gray (Aracnida) Dr. M. B. Malipatil (Hemiptera) Dr. I. D. Naumann (Hymenoptera) Dr. T. R. New (Neuroptera) Dr. T. A. Weir (Coleoptera) The people who provided technical assistance: Holly Ainslie, Dr. Steven Asante, Doreen Beness, Laura Bennett, Samantha Browne, Dr. Mark Coombs, Peter Foreman, Jacqueline Prudon, Sally Schwitzer, Kelly Stanley, Anita Stevenson, Donald Wheatley, and Richard Willis. Dr. Steven Trowell for guidance using serological methods and to Dr. Anne Bourne for her statistical significance. Mr. Robert Gregg for accomodating my family at Tyree whilst sampling in Moree. -

Classical Biological Control of Arthropods in Australia
Classical Biological Contents Control of Arthropods Arthropod index in Australia General index List of targets D.F. Waterhouse D.P.A. Sands CSIRo Entomology Australian Centre for International Agricultural Research Canberra 2001 Back Forward Contents Arthropod index General index List of targets The Australian Centre for International Agricultural Research (ACIAR) was established in June 1982 by an Act of the Australian Parliament. Its primary mandate is to help identify agricultural problems in developing countries and to commission collaborative research between Australian and developing country researchers in fields where Australia has special competence. Where trade names are used this constitutes neither endorsement of nor discrimination against any product by the Centre. ACIAR MONOGRAPH SERIES This peer-reviewed series contains the results of original research supported by ACIAR, or material deemed relevant to ACIAR’s research objectives. The series is distributed internationally, with an emphasis on the Third World. © Australian Centre for International Agricultural Research, GPO Box 1571, Canberra ACT 2601, Australia Waterhouse, D.F. and Sands, D.P.A. 2001. Classical biological control of arthropods in Australia. ACIAR Monograph No. 77, 560 pages. ISBN 0 642 45709 3 (print) ISBN 0 642 45710 7 (electronic) Published in association with CSIRO Entomology (Canberra) and CSIRO Publishing (Melbourne) Scientific editing by Dr Mary Webb, Arawang Editorial, Canberra Design and typesetting by ClarusDesign, Canberra Printed by Brown Prior Anderson, Melbourne Cover: An ichneumonid parasitoid Megarhyssa nortoni ovipositing on a larva of sirex wood wasp, Sirex noctilio. Back Forward Contents Arthropod index General index Foreword List of targets WHEN THE CSIR Division of Economic Entomology, now Commonwealth Scientific and Industrial Research Organisation (CSIRO) Entomology, was established in 1928, classical biological control was given as one of its core activities. -

Downloaded from the Same Database
G C A T T A C G G C A T genes Article Genetic Basis of Maize Resistance to Multiple Insect Pests: Integrated Genome-Wide Comparative Mapping and Candidate Gene Prioritization A. Badji 1,* , D. B. Kwemoi 2, L. Machida 3 , D. Okii 1, N. Mwila 1, S. Agbahoungba 4, F. Kumi 5 , A. Ibanda 1 , A. Bararyenya 1, M. Solemanegy 1, T. Odong 1, P. Wasswa 1, M. Otim 2, G. Asea 2, M. Ochwo-Ssemakula 1, H. Talwana 1, S. Kyamanywa 1 and P. Rubaihayo 1 1 Department of Agricultural Production, Makerere Univesity, P.O. Box 7062 Kampala, Uganda; [email protected] (D.O.); [email protected] (N.M.); [email protected] (A.I.); [email protected] (A.B.); [email protected] (M.S.); [email protected] (T.O.); [email protected] (P.W.); [email protected] (M.O.-S.); [email protected] (H.T.); [email protected] (S.K.); [email protected] (P.R.) 2 Cereals Program, National Crop Resource Research Institute, P.O. Box 7084 Kampala, Uganda; [email protected] (D.B.K.); [email protected] (M.O.); [email protected] (G.A.) 3 Alliance Bioversity International-CIAT, P.O. Box 24384 Kampala, Uganda; [email protected] 4 Laboratory of Applied Ecology, University of Abomey-Calavi, 01BP 526 Cotonou, Benin; [email protected] 5 Department of Crop Science, University of Cape Coast, P.O. Box 5007 PMB Cape Coast, Ghana; [email protected] * Correspondence: [email protected] Received: 19 May 2020; Accepted: 1 June 2020; Published: 24 June 2020 Abstract: Several species of herbivores feed on maize in field and storage setups, making the development of multiple insect resistance a critical breeding target. -

Autographa Gamma
1 Table of Contents Table of Contents Authors, Reviewers, Draft Log 4 Introduction to the Reference 6 Soybean Background 11 Arthropods 14 Primary Pests of Soybean (Full Pest Datasheet) 14 Adoretus sinicus ............................................................................................................. 14 Autographa gamma ....................................................................................................... 26 Chrysodeixis chalcites ................................................................................................... 36 Cydia fabivora ................................................................................................................. 49 Diabrotica speciosa ........................................................................................................ 55 Helicoverpa armigera..................................................................................................... 65 Leguminivora glycinivorella .......................................................................................... 80 Mamestra brassicae....................................................................................................... 85 Spodoptera littoralis ....................................................................................................... 94 Spodoptera litura .......................................................................................................... 106 Secondary Pests of Soybean (Truncated Pest Datasheet) 118 Adoxophyes orana ...................................................................................................... -

Molecular Characterization of Fall Armyworm (Spodoptera Frugiperda) Resistant to Vip3aa20 Protein Expressed in Corn
Molecular Characterization of Fall Armyworm (Spodoptera frugiperda) Resistant to Vip3Aa20 Protein Expressed in Corn Dissertation Presented in partial fulfillment of the requirements for the degree of doctor of philosophy in the Graduate School of The Ohio State University Julio Cesar Fatoretto, B. Sc. Graduate Program in Translational Plant Science The Ohio State University 2017 Dissertation Committee: Andrew P. Michel, Advisor Marcio C. Silva Filho Thomas Mitchell Fernando Consoli Copyright by Julio Cesar Fatoretto 2017 Abstract Transgenic plants containing genes from Bacillus thuringiensis have been used as an alternative to chemical insecticides for insect pest control. The vegetative insecticidal proteins (Vip) secreted during the vegetative growth phase of bacteria are considered a second generation of insecticidal proteins since they do not share any structural or sequence homology with previously used crystal proteins (Cry) as well as having a wide insecticidal spectrum. One of the target pests for this protein is the fall armyworm (FAW) (Spodoptera frugiperda), the most important corn pest in South America. Previously it has been controlled by insecticides and corn expressing Cry proteins, but has rapidly evolved resistance to many control practices and remains a top concern for sustainable biotechnology control efforts. Thus, resistance characterization involving mode of action and genetics of resistance can help with Insect Resistance Management strategies, and improve the durability of control. In this dissertation, using selected FAW population resistant to Vip3Aa20 Bt protein (Vip-R1and Vip-R2) we generated comparative proteomic and transcriptomic data among resistant and susceptible colonies. In the chapter 2, we bring FAW biology/ecology and Brazilian agriculture landscape data to support the high adaptive potential of this pest to genetically modified corn expressing Bt Cry proteins in Brazil. -

Response of Chilo Partellus (Lepidoptera: Crambidae) to Bt Maize in South Africa
Response of Chilo partellus (Lepidoptera: Crambidae) to Bt maize in South Africa J Vorster orcid.org/0000-0001-8126-6860 Dissertation submitted in fulfilment of the requirements for the Masters degree in Environmental Science at the North-West University Supervisor: Prof J van den Berg Co-supervisor: Prof MJ du Plessis Assistant supervisor: Dr A Erasmus Graduation May 2018 23441674 ACKNOWLEDGEMENTS This dissertation would not have been possible without the help of so many people. I am blessed and very grateful to have them in my life. I would like to start with our God Almighty and our Saviour who bestowed upon me the strength, wisdom and peace of mind to finish this project and who also have sent me these blessed people in my life. I would like to thank Prof. Johnnie van den Berg and Dr. Annemie Erasmus for all the guidance and support they have given me. You taught me that small things can make a big difference. Statistics can be difficult sometimes and I thank Prof. Hannalene du Plessis and Prof. Suria Elis for the help with the statistics. Thank you to all the staff at the ARC-GCI that assisted me with the trials in the lab and the planting. Elrine Strydom, Mabel du Toit, Heidi Meyer and Ursula du Plessis, thank you for the countless after hours we had to spend and for the warm hearted kindness you have given me. I would also like to thank my parents whom I dearly love for all the encouragement and motivation to do my best. You taught me that hard work does not come easily, but the fruit that you pick from it is what motivates us. -

Aspects of the Ecology of Helicoverpa Punctigera – What Has Changed?
Chapter 1: Aspects of the ecology of Helicoverpa punctigera – what has changed? 1.1 Background The genus Helicoverpa is part of the noctuid family of moths and has a worldwide range of species, which include Helicoverpa armigera (Hübner, 1805), Helicoverpa assulta (Guenée, 1852) Helicoverpa atacamae (Hardwick, 1965) Helicoverpa fletcheri (Hardwick, 1965), Helicoverpa gelotopoeon (Dyar, 1921), Helicoverpa hardwicki (Matthews, 1999), Helicoverpa hawaiiensis (Quaintance & Brues, 1905), Helicoverpa helenae (Hardwick, 1965), Helicoverpa pallida (Hardwick, 1965), Helicoverpa prepodes (Common, 1985), Helicoverpa punctigera (Wallengren, 1860), Helicoverpa titicaca (Hardwick, 1965), Helicoverpa toddi (Hardwick, 1965) and Helicoverpa zea (Boddie, 1850). As cotton bollworms and legume pod borers, larvae of Helicoverpa spp. are among the most significant crop pests worldwide (Fitt and Cotter, 2005). There are two species of the genus Helicoverpa that are agriculturally significant in Australia, the cotton bollworm Helicoverpa armigera and the native budworm Helicoverpa punctigera. The cosmopolitan H. armigera and the Australian native H. punctigera are the most important pests of field crops in Australia (Zalucki et al., 1986). Although the two species are closely related and share similar colouration and body sizes, each species has differing host-plant ranges, seasonal abundances and geographic ranges (Zalucki et al., 1986, Fitt and Cotter, 2005). Helicoverpa armigera achieves 4-5 generations per season over most of Queensland and much of Northern New South Wales (Fitt and Daly, 1990). The diverse host range of H. armigera means that at any one time there may be a mosaic of subpopulations linked by adult migration, each with different mortality rates and fitness effects imposed by their environment (Dillon et al., 1996). -

Arthropods of Elm Fork Preserve
Arthropods of Elm Fork Preserve Arthropods are characterized by having jointed limbs and exoskeletons. They include a diverse assortment of creatures: Insects, spiders, crustaceans (crayfish, crabs, pill bugs), centipedes and millipedes among others. Column Headings Scientific Name: The phenomenal diversity of arthropods, creates numerous difficulties in the determination of species. Positive identification is often achieved only by specialists using obscure monographs to ‘key out’ a species by examining microscopic differences in anatomy. For our purposes in this survey of the fauna, classification at a lower level of resolution still yields valuable information. For instance, knowing that ant lions belong to the Family, Myrmeleontidae, allows us to quickly look them up on the Internet and be confident we are not being fooled by a common name that may also apply to some other, unrelated something. With the Family name firmly in hand, we may explore the natural history of ant lions without needing to know exactly which species we are viewing. In some instances identification is only readily available at an even higher ranking such as Class. Millipedes are in the Class Diplopoda. There are many Orders (O) of millipedes and they are not easily differentiated so this entry is best left at the rank of Class. A great deal of taxonomic reorganization has been occurring lately with advances in DNA analysis pointing out underlying connections and differences that were previously unrealized. For this reason, all other rankings aside from Family, Genus and Species have been omitted from the interior of the tables since many of these ranks are in a state of flux. -
![Ichneumonid Wasps (Hymenoptera, Ichneumonidae) in the to Scale Caterpillar (Lepidoptera) [1]](https://docslib.b-cdn.net/cover/0863/ichneumonid-wasps-hymenoptera-ichneumonidae-in-the-to-scale-caterpillar-lepidoptera-1-720863.webp)
Ichneumonid Wasps (Hymenoptera, Ichneumonidae) in the to Scale Caterpillar (Lepidoptera) [1]
Central JSM Anatomy & Physiology Bringing Excellence in Open Access Research Article *Corresponding author Bui Tuan Viet, Institute of Ecology an Biological Resources, Vietnam Acedemy of Science and Ichneumonid Wasps Technology, 18 Hoang Quoc Viet, Cau Giay, Hanoi, Vietnam, Email: (Hymenoptera, Ichneumonidae) Submitted: 11 November 2016 Accepted: 21 February 2017 Published: 23 February 2017 Parasitizee a Pupae of the Rice Copyright © 2017 Viet Insect Pests (Lepidoptera) in OPEN ACCESS Keywords the Hanoi Area • Hymenoptera • Ichneumonidae Bui Tuan Viet* • Lepidoptera Vietnam Academy of Science and Technology, Vietnam Abstract During the years 1980-1989,The surveys of pupa of the rice insect pests (Lepidoptera) in the rice field crops from the Hanoi area identified showed that 12 species of the rice insect pests, which were separated into three different groups: I- Group (Stem bore) including Scirpophaga incertulas, Chilo suppressalis, Sesamia inferens; II-Group (Leaf-folder) including Parnara guttata, Parnara mathias, Cnaphalocrocis medinalis, Brachmia sp, Naranga aenescens; III-Group (Bite ears) including Mythimna separata, Mythimna loryei, Mythimna venalba, Spodoptera litura . From these organisms, which 15 of parasitoid species were found, those species belonging to 5 families in of the order Hymenoptera (Ichneumonidae, Chalcididae, Eulophidae, Elasmidae, Pteromalidae). Nine of these, in which there were 9 of were ichneumonid wasp species: Xanthopimpla flavolineata, Goryphus basilaris, Xanthopimpla punctata, Itoplectis naranyae, Coccygomimus nipponicus, Coccygomimus aethiops, Phaeogenes sp., Atanyjoppa akonis, Triptognatus sp. We discuss the general biology, habitat preferences, and host association of the knowledge of three of these parasitoids, (Xanthopimpla flavolineata, Phaeogenes sp., and Goryphus basilaris). Including general biology, habitat preferences and host association were indicated and discussed. -

Morphology of the Immatures and Biology of Chinavia Longicorialis (Breddin) (Hemiptera: Pentatomidae)
74 January - February 2009 SYSTEMATICS, MORPHOLOGY AND PHYSIOLOGY Morphology of the Immatures and Biology of Chinavia longicorialis (Breddin) (Hemiptera: Pentatomidae) VIVIANA C MATESCO, CRISTIANO F SCHWERTNER, JOCELIA GRAZIA Programa de Pós-Graduação em Biologia Animal, Depto. Zoologia, IB, Univ. Federal do Rio Grande do Sul, Av. Bento Gonçalves, 9500, Bloco IV, Pr. 43435, Sl. 216, Porto Alegre, RS, 91501-970; [email protected]; acrosternum@ yahoo.com.br; [email protected]; Contribuição nº 570 do Depto. Zoologia – UFRGS Edited by Fernando Barbosa Noll – UNESP Neotropical Entomology 38(1):074-082 (2009) Morfologia dos Estágios Imaturos e Biologia de Chinavia longicorialis (Breddin) (Hemiptera: Pentatomidae) RESUMO - Chinavia longicorialis (Breddin) é encontrada apenas no Brasil, Argentina e Uruguai, sobre plantas hospedeiras de pelo menos três famílias diferentes. Adultos e ninfas dessa espécie foram coletados no Parque Estadual do Espinilho (Barra do Quaraí, RS) e na região da Serra do Sudeste (Canguçu e Caçapava do Sul, RS) e mantidos sob condições controladas (24 ± 1°C; UR 70 ± 10%; 12hL:12hE), alimentados com vagens verdes de feijão (Phaseolus vulgaris L.). Os ovos e as ninfas de primeiro ínstar de C. longicorialis são muito semelhantes àqueles das demais espécies de Chinavia; porém apresentando manchas alaranjadas na margem lateral dos segmentos torácicos. As manchas abdominais, a partir do terceiro ínstar, são nitidamente divididas pelas pseudo-suturas do abdome, o que constitui caráter diagnóstico para C. longicorialis. Ninfas do terceiro ao quinto ínstar apresentaram formas claras e escuras. Não foi observada sobreposição nas medidas de largura da cabeça entre diferentes estádios. O número de ovos por postura mais freqüente foi 14; sugere-se a adoção da moda como melhor estimativa para o tamanho das posturas em Pentatomidae. -
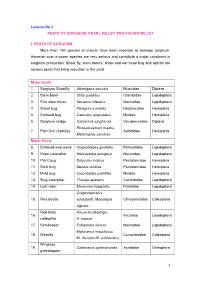
Lecture No 3 PESTS of SORGHUM, PEARL MILLET and FINGER MILLET
Lecture No 3 PESTS OF SORGHUM, PEARL MILLET AND FINGER MILLET I. PESTS OF SORGHUM More than 150 species of insects have been reported to damage sorghum. However over a dozen species are very serious and constitute a major constraint in sorghum production. Shoot fly, stem borers, shoot and ear head bug and aphids are serious pests that bring reduction in the yield. Major pests 1. Sorghum Shootfly Atherigona soccata Muscidae Diptera 2. Stem borer Chilo partellus Crambidae Lepidoptera 3. Pink stem borer Sesamia inferens Noctuidae Lepidoptera 4 Shoot bug Peregrinus maidis Delphacidae Hemiptera 5. Earhead bug Calocoris angustatus Miridae Hemiptera 6. Sorghum midge Contarinia sorghicola Cecidomyiidae Diptera Rhopalosiphum maidis, 7. Plant lice (Aphids) Aphididae Hemiptera Melanaphis sacchari Minor Pests 8. Earhead web worm Cryptoblabes gnidiella Pyraustidae Lepidoptera 9. Gram caterpillar Helicoverpa armigera Noctuidae Lepidoptera 10. Plant bug Dolycoris indicus Pentatomidae Hemiptera 11. Stink bug Nezara viridula Pentatomidae Hemiptera 12. Mirid bug Creontiades pallidifer Miridae Hemiptera 13. Slug caterpillar Thosea apierens Cochlididae Lepidoptera 14. Leaf roller Marasmia trapezalis Pyralidae Lepidoptera Cryptocephalus 15. Flea beetle schestedii, Monolepta Chrysomelidae Coleoptera signata Red hairy Amsacta albistriga, 16. Arctiidae Lepidoptera caterpillar A. moorei 17. Semilooper Eublemma silicula Noctuidae Lepidoptera Myllocerus maculosus 18. Weevils Curculionidae Coleoptera M. discolor,M. subfaciatus Wingless 19. Colemania sphenaroides Acrididae Orthoptera grasshopper MAJOR PESTS 1.Sorghum Shootfly: Atherigona soccata (Muscidae: Diptera) Distribution and status Maharashtra, Andhra Pradesh, Tamil Nadu and Karnataka Host range: Maize, ragi, bajra, rice, wheat and grasses Damage symptoms The maggot on hatching migrates to the upper surface of leaf and enters between the leaf sheath and stem. -

Heteroptera: Hemiptera ) from Chhattisgarh, India
BISWAS et al.: On an account of Pentatomoidea.....from Chhattisgarh, India ISSN 0375-1511211 Rec. zool. Surv. India : 114(Part-2) : 211-231, 2014 ON AN ACCOUNT OF PENTATOMOIDEA (HETEROPTERA: HEMIPTERA ) FROM CHHATTISGARH, INDIA B. BISWAS, M. E. HASSAN, KAILASH CHANDRA, SANDEEP KUSHWAHA** AND PARAMITA MUKHERJEE Zoological Survey of India, M-Block, New Alipore, Kolkata-700053, India ** Zoological Survey of India, Central Zone Regional Centre, Vijay Nagar, Jabalpur-482002 INTRODUCTION SYSTEMATIC ACCOUNT The pentatomids are commonly known as Family I PENTATOMIDAE “shield bugs” or “stink bugs” as their bodies are Subfamily PENTATOMINAE usually covered by a shield shaped scutellum covering more than half of the abdomen, tibia with Tribe ANTESTINI weak or no spine, 5 segmented antennae which Genus 1. Antestia Stal, 1864 gives its family name and most of them emit an 1. Antestia anchora (Thunberg) unpleasant odour, offensive in nature, produced by a pair of glands in the thorax and is released through *2. Antestia cruciata (Fabricius) openings in the metathorax. Although majority Genus 2. Plautia Stal, 1867 of these bugs are plant sucking, the members *3. Plautia crossota (Fabricius) belonging to the family Asopinae are wholly or partially predaceous. Pentatomoidea is one of the Tribe AGONOSCELIDINI largest superfamilies of Heteroptera comprising of Genus 3. Agonoscelis Spin, 1837 1301 genera and 7182 species distributed in sixteen 4. Agonoscelis nubilis (Fabricius) families all over the world (Henry, 2009). Of these, family Pentatomidae alone represents 896 genera Tribe CARPOCORINI and 4722 species distributed in eight subfamilies Genus 4. Gulielmus Distant, 1901 (Pentatominae, Asopinae, Podopinae, Edessinae, 5. Gulielmus laterarius Distant Phyllocephalinae, Discocephalinae, Cyrtocorinae and Serbaninae).