Aprostocetus (Ootetrastichus) Theioneurus (Masi) (Hymenoptera
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arthropod Containment in Plant Research
Arthropod Containment in Plant Research Jian J Duan & Jay Bancroft USDA ARS Beneficial Insects Research Unit Newark, Delaware What we do at USDA ARS BIIRU - • To develop biological control programs against invasive (non-native) agriculture and forest pests – Research involves both the plant-feeding insects and their natural enemies (predators & parasitoids) Invasive Emerald Ash Borer Larval Parasitoids of EAB (EAB) The Goal of Insect Containment at USDA ARS BIIRU-Quarantine Facility • Prevent “accidental introduction” of “unwanted” non-native insects from damaging our agriculture and forestry Outlines • Why do we need to contain insects in plant research? • How can we most effectively contain insects in plant research? • Quarantine containment facility and standard operation procedures Why Do We Need To Contain Insects in Plant Research • Non-native insects can become serious invasive pests in a newly introduced region because disassociation with co- evolved natural enemies • Non-native insects used in plant research should be contained prior to regulatory approval for environmental releases Non-native, plant-feeding insects can become devastating pests in agriculture and forestry Detected in Michigan in 2002 • 31 States in the U.S. • Killed millions of ash trees Emerald Ash Borer Native Range of EAB & Origin of EAB-Parasitoids Origin of EAB Biocontrol Agents (Year releases began in US) 1. Oobius agrili 2. Tetrastichus planipennisi 3. Spathius agrili 4. Spathius galinae Russia China 1 4 2 3 Prevent “accidental introduction” of weed biocontrol -

Effect of Temperature on Life History of Quadrastichus Haitiensis
Biological Control 36 (2006) 189–196 www.elsevier.com/locate/ybcon Effect of temperature on life history of Quadrastichus haitiensis (Hymenoptera: Eulophidae), an endoparasitoid of Diaprepes abbreviatus (Coleoptera: Curculionidae) J. Castillo a, J.A. Jacas b,*, J.E. Pen˜a a, B.J. Ulmer a, D.G. Hall c a University of Florida/IFAS, Tropical Research and Education Center, 18905 SW 280th Street, Homestead, FL 33031, USA b Departament de Cie`ncies Experimentals, Universitat Jaume I, Campus del Riu Sec, E-12071-Castello´ de la Plana, Spain c USDA, USRHL, Fort Pierce, FL 34945, USA Received 9 May 2005; accepted 26 September 2005 Available online 13 December 2005 Abstract The influence of temperature on life history traits of the egg parasitoid Quadrastichus haitiensis (Gahan) (Hymenoptera: Eulophidae) was investigated in the laboratory on eggs of the root weevil Diaprepes abbreviatus (L.) (Coleoptera: Curculionidae). Duration of devel- opment from egg to adult decreased from 39.99 ± 0.27 days to 13.57 ± 0.07 days (mean ± SE) as temperature increased from 20 to 33 °C, respectively. No development was observed at 5–15 °C. Fecundity was highest at 25 and 30 °C (70–73 eggs per female) but was reduced at 33 °C (21.5 eggs per female). Oviposition rate was also reduced at 33 °C. Q. haitiensis accepted host eggs from 0 to 7 days old for oviposition but was most prolific when parasitizing 1- to 4-day-old eggs. Very few adult Q. haitiensis emerged from host eggs that were 5–7 days old, however, D. abbreviatus egg mortality was similar for eggs 0–7 days old. -

Downloaded from the Same Database
G C A T T A C G G C A T genes Article Genetic Basis of Maize Resistance to Multiple Insect Pests: Integrated Genome-Wide Comparative Mapping and Candidate Gene Prioritization A. Badji 1,* , D. B. Kwemoi 2, L. Machida 3 , D. Okii 1, N. Mwila 1, S. Agbahoungba 4, F. Kumi 5 , A. Ibanda 1 , A. Bararyenya 1, M. Solemanegy 1, T. Odong 1, P. Wasswa 1, M. Otim 2, G. Asea 2, M. Ochwo-Ssemakula 1, H. Talwana 1, S. Kyamanywa 1 and P. Rubaihayo 1 1 Department of Agricultural Production, Makerere Univesity, P.O. Box 7062 Kampala, Uganda; [email protected] (D.O.); [email protected] (N.M.); [email protected] (A.I.); [email protected] (A.B.); [email protected] (M.S.); [email protected] (T.O.); [email protected] (P.W.); [email protected] (M.O.-S.); [email protected] (H.T.); [email protected] (S.K.); [email protected] (P.R.) 2 Cereals Program, National Crop Resource Research Institute, P.O. Box 7084 Kampala, Uganda; [email protected] (D.B.K.); [email protected] (M.O.); [email protected] (G.A.) 3 Alliance Bioversity International-CIAT, P.O. Box 24384 Kampala, Uganda; [email protected] 4 Laboratory of Applied Ecology, University of Abomey-Calavi, 01BP 526 Cotonou, Benin; [email protected] 5 Department of Crop Science, University of Cape Coast, P.O. Box 5007 PMB Cape Coast, Ghana; [email protected] * Correspondence: [email protected] Received: 19 May 2020; Accepted: 1 June 2020; Published: 24 June 2020 Abstract: Several species of herbivores feed on maize in field and storage setups, making the development of multiple insect resistance a critical breeding target. -

Hymenoptera: Eulophidae) 321-356 ©Entomofauna Ansfelden/Austria; Download Unter
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Entomofauna Jahr/Year: 2007 Band/Volume: 0028 Autor(en)/Author(s): Yefremova Zoya A., Ebrahimi Ebrahim, Yegorenkova Ekaterina Artikel/Article: The Subfamilies Eulophinae, Entedoninae and Tetrastichinae in Iran, with description of new species (Hymenoptera: Eulophidae) 321-356 ©Entomofauna Ansfelden/Austria; download unter www.biologiezentrum.at Entomofauna ZEITSCHRIFT FÜR ENTOMOLOGIE Band 28, Heft 25: 321-356 ISSN 0250-4413 Ansfelden, 30. November 2007 The Subfamilies Eulophinae, Entedoninae and Tetrastichinae in Iran, with description of new species (Hymenoptera: Eulophidae) Zoya YEFREMOVA, Ebrahim EBRAHIMI & Ekaterina YEGORENKOVA Abstract This paper reflects the current degree of research of Eulophidae and their hosts in Iran. A list of the species from Iran belonging to the subfamilies Eulophinae, Entedoninae and Tetrastichinae is presented. In the present work 47 species from 22 genera are recorded from Iran. Two species (Cirrospilus scapus sp. nov. and Aprostocetus persicus sp. nov.) are described as new. A list of 45 host-parasitoid associations in Iran and keys to Iranian species of three genera (Cirrospilus, Diglyphus and Aprostocetus) are included. Zusammenfassung Dieser Artikel zeigt den derzeitigen Untersuchungsstand an eulophiden Wespen und ihrer Wirte im Iran. Eine Liste der für den Iran festgestellten Arten der Unterfamilien Eu- lophinae, Entedoninae und Tetrastichinae wird präsentiert. Mit vorliegender Arbeit werden 47 Arten in 22 Gattungen aus dem Iran nachgewiesen. Zwei neue Arten (Cirrospilus sca- pus sp. nov. und Aprostocetus persicus sp. nov.) werden beschrieben. Eine Liste von 45 Wirts- und Parasitoid-Beziehungen im Iran und ein Schlüssel für 3 Gattungen (Cirro- spilus, Diglyphus und Aprostocetus) sind in der Arbeit enthalten. -

Redalyc.Distribution and Host Records of Melittobia (Hymenoptera: Eulophidae) from Mexico
Revista Mexicana de Biodiversidad ISSN: 1870-3453 [email protected] Universidad Nacional Autónoma de México México González, Jorge M.; Matthews, Robert W.; Bradleigh Vinson, S. Distribution and host records of Melittobia (Hymenoptera: Eulophidae) from Mexico Revista Mexicana de Biodiversidad, vol. 79, núm. 2, diciembre, 2008, pp. 529-531 Universidad Nacional Autónoma de México Distrito Federal, México Available in: http://www.redalyc.org/articulo.oa?id=42511935026 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Revista Mexicana de Biodiversidad 79: 529- 531, 2008 Nota Científi ca Distribution and host records of Melittobia (Hymenoptera: Eulophidae) from Mexico Distribución y huéspedes de Melittobia (Hymenoptera: Eulophidae) en México Jorge M. González1*, Robert W. Matthews2 and S. Bradleigh Vinson1 1Texas A & M. University, Department of Entomology, Entomology Research Laboratory, College Station, Texas 77843-2475, USA. 2University of Georgia, Department of Entomology, Athens, Georgia 30602, USA. *Correspondent: [email protected] Abstract. Specimens of a parasitoid wasp attacking pupae of Anastrepha ludens (Loew) in Mexico were identifi ed as Melittobia digitata Dahms, and after a revision of several worldwide insect collections, M. australica Girault was also found to be present in Mexico. Distribution, diagnosis, hosts and collection locations are given for both species. The possibility that M. acasta (Walker) is also present in Mexico is discussed. Key words: Eulophidae, Melittobia australica, M. digitata, M. acasta, Anastrepha ludens, Mexico, distribution. Resumen. Se identifi caron como Melittobia digitata Dahms ejemplares de un parasitoide atacando pupas de Anastrepha ludens (Loew) proveniente de México. -

The Entomofauna on Eucalyptus in Israel: a Review
EUROPEAN JOURNAL OF ENTOMOLOGYENTOMOLOGY ISSN (online): 1802-8829 Eur. J. Entomol. 116: 450–460, 2019 http://www.eje.cz doi: 10.14411/eje.2019.046 REVIEW The entomofauna on Eucalyptus in Israel: A review ZVI MENDEL and ALEX PROTASOV Department of Entomology, Institute of Plant Protection, Agricultural Research Organization, The Volcani Center, Rishon LeTzion 7528809, Israel; e-mails: [email protected], [email protected] Key words. Eucalyptus, Israel, invasive species, native species, insect pests, natural enemies Abstract. The fi rst successful Eucalyptus stands were planted in Israel in 1884. This tree genus, particularly E. camaldulensis, now covers approximately 11,000 ha and constitutes nearly 4% of all planted ornamental trees. Here we review and discuss the information available about indigenous and invasive species of insects that develop on Eucalyptus trees in Israel and the natural enemies of specifi c exotic insects of this tree. Sixty-two phytophagous species are recorded on this tree of which approximately 60% are indigenous. The largest group are the sap feeders, including both indigenous and invasive species, which are mostly found on irrigated trees, or in wetlands. The second largest group are wood feeders, polyphagous Coleoptera that form the dominant native group, developing in dying or dead wood. Most of the seventeen parasitoids associated with the ten invasive Eucalyptus-specifi c species were introduced as biocontrol agents in classical biological control projects. None of the polyphagous species recorded on Eucalyptus pose any threat to this tree. The most noxious invasive specifi c pests, the gall wasps (Eulophidae) and bronze bug (Thaumastocoris peregrinus), are well controlled by introduced parasitoids. -

Response of Chilo Partellus (Lepidoptera: Crambidae) to Bt Maize in South Africa
Response of Chilo partellus (Lepidoptera: Crambidae) to Bt maize in South Africa J Vorster orcid.org/0000-0001-8126-6860 Dissertation submitted in fulfilment of the requirements for the Masters degree in Environmental Science at the North-West University Supervisor: Prof J van den Berg Co-supervisor: Prof MJ du Plessis Assistant supervisor: Dr A Erasmus Graduation May 2018 23441674 ACKNOWLEDGEMENTS This dissertation would not have been possible without the help of so many people. I am blessed and very grateful to have them in my life. I would like to start with our God Almighty and our Saviour who bestowed upon me the strength, wisdom and peace of mind to finish this project and who also have sent me these blessed people in my life. I would like to thank Prof. Johnnie van den Berg and Dr. Annemie Erasmus for all the guidance and support they have given me. You taught me that small things can make a big difference. Statistics can be difficult sometimes and I thank Prof. Hannalene du Plessis and Prof. Suria Elis for the help with the statistics. Thank you to all the staff at the ARC-GCI that assisted me with the trials in the lab and the planting. Elrine Strydom, Mabel du Toit, Heidi Meyer and Ursula du Plessis, thank you for the countless after hours we had to spend and for the warm hearted kindness you have given me. I would also like to thank my parents whom I dearly love for all the encouragement and motivation to do my best. You taught me that hard work does not come easily, but the fruit that you pick from it is what motivates us. -

Redalyc.Interactions Among Host Plants, Lepidoptera Leaf Miners And
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Yefremova, Z. A.; Kravchenko, V. D. Interactions among host plants, Lepidoptera leaf miners and their parasitoids in the forest- steppe zone of Russia (Insecta: Lepidoptera, Hymenoptera) SHILAP Revista de Lepidopterología, vol. 43, núm. 170, junio, 2015, pp. 271-280 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45541421012 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative 271-280 Interactions among host 3/6/15 10:45 Página 271 SHILAP Revta. lepid., 43 (170), junio 2015: 271-280 eISSN: 2340-4078 ISSN: 0300-5267 Interactions among host plants, Lepidoptera leaf miners and their parasitoids in the forest-steppe zone of Russia (Insecta: Lepidoptera, Hymenoptera) Z. A. Yefremova & V. D. Kravchenko Abstract The article reports on the quantitative description of the food web structure of the community consisting of 65 species of Lepidoptera leaf miners reared from 34 plant species, as well as 107 species of parasitoid eulophid wasps (Hymenoptera: Eulophidae). The study was conducted in the forest-steppe zone of the Middle Volga in Russia over 13 years (2000-2012). Leaf miners have been found to be highly host plant-specific. Most of them are associated with only one or two plant species and therefore the number of links between trophic levels is 73, which is close to the total number of Lepidoptera species (linkage density is 1.12). -

Early-Season Phenology and Temporal Dynamics of the Common Asparagus Beetle, Crioceris Asparagi (Coleoptera: Chrysomelidae), in Southern Minnesota
The Great Lakes Entomologist Volume 39 Numbers 1 & 2 - Spring/Summer 2006 Numbers Article 9 1 & 2 - Spring/Summer 2006 April 2006 Early-Season Phenology and Temporal Dynamics of the Common Asparagus Beetle, Crioceris Asparagi (Coleoptera: Chrysomelidae), in Southern Minnesota Suzanne J. Wold-Burkness University of Minnesota P. C. Bolin University of Minnesota W. D. Hutchison University of Minnesota Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation Wold-Burkness, Suzanne J.; Bolin, P. C.; and Hutchison, W. D. 2006. "Early-Season Phenology and Temporal Dynamics of the Common Asparagus Beetle, Crioceris Asparagi (Coleoptera: Chrysomelidae), in Southern Minnesota," The Great Lakes Entomologist, vol 39 (1) Available at: https://scholar.valpo.edu/tgle/vol39/iss1/9 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. Wold-Burkness et al.: Early-Season Phenology and Temporal Dynamics of the Common Aspara 2 THE GREAT LAKES ENTOMOLOGIST Vol. 39, Nos. & 2 EARLY-SEASON PHENOLOGY AND TEMPORAL DYNAMICS OF THE COMMON ASPARAGUS BEETLE, CRIOCERIS ASPARAGI (COLEOPTERA: CHRYSOMELIDAE), IN SOUTHERN MINNESOTA Suzanne J. Wold-Burkness, P. C. Bolin, and W. D. Hutchison ABSTRACT During the years 1991-1994, studies were conducted to determine the early-season phenology and temporal dynamics of Crioceris asparagi (L.) (Co- leoptera: Chrysomelidae) in southern Minnesota asparagus. To document the early-season phenology, asparagus plots were sampled for egg, larval, and adult stages of C. -

Zootaxa, a New Genus and Species of Tetrastichinae (Hymenoptera: Eulophidae)
Zootaxa 1745: 63–68 (2008) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2008 · Magnolia Press ISSN 1175-5334 (online edition) A new genus and species of Tetrastichinae (Hymenoptera: Eulophidae) inducing galls in seed capsules of Eucalyptus IL-KWON KIM & JOHN LA SALLE CSIRO Entomology, GPO Box 1700, Canberra, ACT 2601, Australia School of Botany and Zoology; The Australian National University, Canberra, ACT, 0200, Australia. E-mail: [email protected] Abstract Leprosa milga Kim & La Salle gen. & sp. nov. (Hymenoptera: Eulophidae: Tetrastichinae) is described from Eucalyptus seed capsules. The new species is an Australian seed gall inducer which has become established in South Africa and Italy. The relationship of Leprosa to two other genera of seed gall inducing tetrastichines, Quadrastichodella and Moona, is discussed. Key words: Tetrastichinae, Leprosa, Quadrastichodella, Moona, Eucalyptus Introduction Most members of Tetrastichinae are known to be entomophagous as primary or hyper parasitoids on a wide variety of insects, as well as a few other arthropod hosts, including even spider egg sacs and nematodes (La Salle 1994). However, several species are known to be phytophagous, and these include gall inducers (La Salle 2005). Within Australia, tetrastichine gall-inducers mainly attack Eucalyptus, and a few other Myrta- ceae, and can induce galls on twigs, leaves, flower buds and seeds (Bouček 1988; Headrick et al. 1995; Noyes 2002, 2003; Kim et al. 2004; Kim et al. 2005; La Salle 2005). The vast majority of Australian tetrastichines are parasitoids, and many of them are associated with galls as inquilines or parasitoids (Bouček, 1988; La Salle 2005). -

Extreme Diversity of Tropical Parasitoid Wasps Exposed by Iterative Integration of Natural History, DNA Barcoding, Morphology, and Collections
Extreme diversity of tropical parasitoid wasps exposed by iterative integration of natural history, DNA barcoding, morphology, and collections M. Alex Smith*†, Josephine J. Rodriguez‡, James B. Whitfield‡, Andrew R. Deans§, Daniel H. Janzen†¶, Winnie Hallwachs¶, and Paul D. N. Hebert* *The Biodiversity Institute of Ontario, University of Guelph, Guelph Ontario, N1G 2W1 Canada; ‡Department of Entomology, 320 Morrill Hall, University of Illinois, 505 S. Goodwin Avenue, Urbana, IL 61801; §Department of Entomology, North Carolina State University, Campus Box 7613, 2301 Gardner Hall, Raleigh, NC 27695-7613; and ¶Department of Biology, University of Pennsylvania, Philadelphia, PA 19104-6018 Contributed by Daniel H. Janzen, May 31, 2008 (sent for review April 18, 2008) We DNA barcoded 2,597 parasitoid wasps belonging to 6 microgas- A detailed recognition of species in parasitoid communities is trine braconid genera reared from parapatric tropical dry forest, cloud necessary because of the pivotal role parasitoids play in food web forest, and rain forest in Area de Conservacio´ n Guanacaste (ACG) in structure and dynamics. While generalizations about the effects of northwestern Costa Rica and combined these data with records of parasitoids on community diversity are complex (7), a common- caterpillar hosts and morphological analyses. We asked whether place predictor of the impact of a parasitoid species on local host barcoding and morphology discover the same provisional species and dynamics is whether the parasitoid is a generalist or specialist. A whether the biological entities revealed by our analysis are congruent generalist, especially a mobile one, is viewed as stabilizing food webs with wasp host specificity. Morphological analysis revealed 171 (see ref. -
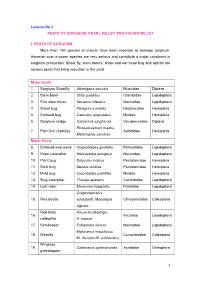
Lecture No 3 PESTS of SORGHUM, PEARL MILLET and FINGER MILLET
Lecture No 3 PESTS OF SORGHUM, PEARL MILLET AND FINGER MILLET I. PESTS OF SORGHUM More than 150 species of insects have been reported to damage sorghum. However over a dozen species are very serious and constitute a major constraint in sorghum production. Shoot fly, stem borers, shoot and ear head bug and aphids are serious pests that bring reduction in the yield. Major pests 1. Sorghum Shootfly Atherigona soccata Muscidae Diptera 2. Stem borer Chilo partellus Crambidae Lepidoptera 3. Pink stem borer Sesamia inferens Noctuidae Lepidoptera 4 Shoot bug Peregrinus maidis Delphacidae Hemiptera 5. Earhead bug Calocoris angustatus Miridae Hemiptera 6. Sorghum midge Contarinia sorghicola Cecidomyiidae Diptera Rhopalosiphum maidis, 7. Plant lice (Aphids) Aphididae Hemiptera Melanaphis sacchari Minor Pests 8. Earhead web worm Cryptoblabes gnidiella Pyraustidae Lepidoptera 9. Gram caterpillar Helicoverpa armigera Noctuidae Lepidoptera 10. Plant bug Dolycoris indicus Pentatomidae Hemiptera 11. Stink bug Nezara viridula Pentatomidae Hemiptera 12. Mirid bug Creontiades pallidifer Miridae Hemiptera 13. Slug caterpillar Thosea apierens Cochlididae Lepidoptera 14. Leaf roller Marasmia trapezalis Pyralidae Lepidoptera Cryptocephalus 15. Flea beetle schestedii, Monolepta Chrysomelidae Coleoptera signata Red hairy Amsacta albistriga, 16. Arctiidae Lepidoptera caterpillar A. moorei 17. Semilooper Eublemma silicula Noctuidae Lepidoptera Myllocerus maculosus 18. Weevils Curculionidae Coleoptera M. discolor,M. subfaciatus Wingless 19. Colemania sphenaroides Acrididae Orthoptera grasshopper MAJOR PESTS 1.Sorghum Shootfly: Atherigona soccata (Muscidae: Diptera) Distribution and status Maharashtra, Andhra Pradesh, Tamil Nadu and Karnataka Host range: Maize, ragi, bajra, rice, wheat and grasses Damage symptoms The maggot on hatching migrates to the upper surface of leaf and enters between the leaf sheath and stem.