Atcveljavni.Xlsx
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comparison of Norepinephrine and Cafedrine/Theodrenaline Regimens for Maintaining Maternal Blood Pressure During
IBIMA Publishing Obstetrics & Gynecology: An International Journal http://www.ibimapublishing.com/journals/GYNE/gyne.html Vol. 2015 (2015), Article ID 714966, 12 pages DOI: 10.5171/2015.714966 Research Article Comparison of Norepinephrine and Cafedrine/Theodrenaline Regimens for Maintaining Maternal Blood Pressure during Spinal Anaesthesia for Caesarean Section Hoyme, M.1, Scheungraber, C.2, Reinhart, K.3 and Schummer, W.3 1Department of Internal Medicine I (Cardiology, Angiology, Pneumology) Friedrich Schiller University, Jena, Germany 2Departments of Obstetrics and Gynecology, Friedrich Schiller University, Jena, Germany 3Clinic of Anaesthesiology and Intensive Care Medicine, Friedrich Schiller University, Jena, Germany Correspondence should be addressed to: Schummer, W.; [email protected] Received date: 9 January 2014; Accepted date: 4 April 2014; Published date: 26 August 2015 Academic Editor: Dan Benhamou Copyright © 2015. Hoyme, M., Scheungraber, C., Reinhart, K. and Schummer, W. Distributed under Creative Commons CC-BY 4.0 Abstract Common phenomena of spinal anaesthesia for caesarean sections are hypotension and cardiovascular depression, both of which require immediate action. Akrinor® (theodrenaline and cafedrine), a sympathomimetic agent commonly used in Germany for such cases, was phased out with little notice at the end of 2005. Phenylephrine was not and is not an approved drug. Norepinephrine became the first-line drug. The outcome in neonates delivered by elective caesarean section under spinal anaesthesia was studied. At our university hospital, all elective caesarean sections under spinal anaesthesia from 2005 and 2006 were analysed regarding hypotension and the vasopressor administered. If maternal arterial pressure decreased by more than 20% of baseline, patients in 2005 received Akrinor®; patients in 2006 received norepinephrine. -

Hypotension After Spinal Anesthesia for Cesarean
REVIEW CURRENT OPINION Hypotension after spinal anesthesia for cesarean section: how to approach the iatrogenic sympathectomy Christina Massotha, Lisa To¨pelb, and Manuel Wenkb Purpose of review Hypotension during cesarean section remains a frequent complication of spinal anesthesia and is associated with adverse maternal and fetal events. Recent findings Despite ongoing research, no single measure for sufficient treatment of spinal-induced hypotension was 05/25/2020 on BhDMf5ePHKbH4TTImqenVDezntqwKeJGjCYTUOBBz0EyVSh+WquM7uJo3U//pWTO by http://journals.lww.com/co-anesthesiology from Downloaded Downloaded identified so far. Current literature discusses the efficacy of low-dose spinal anesthesia, timing and solutions for adequate fluid therapy and various vasopressor regimens. Present guidelines favor the use of from phenylephrine over ephedrine because of decreased umbilical cord pH values, while norepinephrine is http://journals.lww.com/co-anesthesiology discussed as a probable superior alternative with regard to maternal bradycardia, although supporting data is limited. Alternative pharmacological approaches, such as 5HT3-receptor antagonists and physical methods may be taken into consideration to further improve hemodynamic stability. Summary Current evidence favors a combined approach of low-dose spinal anesthesia, adequate fluid therapy and vasopressor support to address maternal spinal-induced hypotension. As none of the available vasopressors by is associated with relevantly impaired maternal and fetal outcomes, none of them should -

Phosphodiesterase (PDE)
Phosphodiesterase (PDE) Phosphodiesterase (PDE) is any enzyme that breaks a phosphodiester bond. Usually, people speaking of phosphodiesterase are referring to cyclic nucleotide phosphodiesterases, which have great clinical significance and are described below. However, there are many other families of phosphodiesterases, including phospholipases C and D, autotaxin, sphingomyelin phosphodiesterase, DNases, RNases, and restriction endonucleases, as well as numerous less-well-characterized small-molecule phosphodiesterases. The cyclic nucleotide phosphodiesterases comprise a group of enzymes that degrade the phosphodiester bond in the second messenger molecules cAMP and cGMP. They regulate the localization, duration, and amplitude of cyclic nucleotide signaling within subcellular domains. PDEs are therefore important regulators ofsignal transduction mediated by these second messenger molecules. www.MedChemExpress.com 1 Phosphodiesterase (PDE) Inhibitors, Activators & Modulators (+)-Medioresinol Di-O-β-D-glucopyranoside (R)-(-)-Rolipram Cat. No.: HY-N8209 ((R)-Rolipram; (-)-Rolipram) Cat. No.: HY-16900A (+)-Medioresinol Di-O-β-D-glucopyranoside is a (R)-(-)-Rolipram is the R-enantiomer of Rolipram. lignan glucoside with strong inhibitory activity Rolipram is a selective inhibitor of of 3', 5'-cyclic monophosphate (cyclic AMP) phosphodiesterases PDE4 with IC50 of 3 nM, 130 nM phosphodiesterase. and 240 nM for PDE4A, PDE4B, and PDE4D, respectively. Purity: >98% Purity: 99.91% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 1 mg, 5 mg Size: 10 mM × 1 mL, 10 mg, 50 mg (R)-DNMDP (S)-(+)-Rolipram Cat. No.: HY-122751 ((+)-Rolipram; (S)-Rolipram) Cat. No.: HY-B0392 (R)-DNMDP is a potent and selective cancer cell (S)-(+)-Rolipram ((+)-Rolipram) is a cyclic cytotoxic agent. (R)-DNMDP, the R-form of DNMDP, AMP(cAMP)-specific phosphodiesterase (PDE) binds PDE3A directly. -

Prohibited Substances List
Prohibited Substances List This is the Equine Prohibited Substances List that was voted in at the FEI General Assembly in November 2009 alongside the new Equine Anti-Doping and Controlled Medication Regulations(EADCMR). Neither the List nor the EADCM Regulations are in current usage. Both come into effect on 1 January 2010. The current list of FEI prohibited substances remains in effect until 31 December 2009 and can be found at Annex II Vet Regs (11th edition) Changes in this List : Shaded row means that either removed or allowed at certain limits only SUBSTANCE ACTIVITY Banned Substances 1 Acebutolol Beta blocker 2 Acefylline Bronchodilator 3 Acemetacin NSAID 4 Acenocoumarol Anticoagulant 5 Acetanilid Analgesic/anti-pyretic 6 Acetohexamide Pancreatic stimulant 7 Acetominophen (Paracetamol) Analgesic/anti-pyretic 8 Acetophenazine Antipsychotic 9 Acetylmorphine Narcotic 10 Adinazolam Anxiolytic 11 Adiphenine Anti-spasmodic 12 Adrafinil Stimulant 13 Adrenaline Stimulant 14 Adrenochrome Haemostatic 15 Alclofenac NSAID 16 Alcuronium Muscle relaxant 17 Aldosterone Hormone 18 Alfentanil Narcotic 19 Allopurinol Xanthine oxidase inhibitor (anti-hyperuricaemia) 20 Almotriptan 5 HT agonist (anti-migraine) 21 Alphadolone acetate Neurosteriod 22 Alphaprodine Opiod analgesic 23 Alpidem Anxiolytic 24 Alprazolam Anxiolytic 25 Alprenolol Beta blocker 26 Althesin IV anaesthetic 27 Althiazide Diuretic 28 Altrenogest (in males and gelidngs) Oestrus suppression 29 Alverine Antispasmodic 30 Amantadine Dopaminergic 31 Ambenonium Cholinesterase inhibition 32 Ambucetamide Antispasmodic 33 Amethocaine Local anaesthetic 34 Amfepramone Stimulant 35 Amfetaminil Stimulant 36 Amidephrine Vasoconstrictor 37 Amiloride Diuretic 1 Prohibited Substances List This is the Equine Prohibited Substances List that was voted in at the FEI General Assembly in November 2009 alongside the new Equine Anti-Doping and Controlled Medication Regulations(EADCMR). -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

BMJ Open Is Committed to Open Peer Review. As Part of This Commitment We Make the Peer Review History of Every Article We Publish Publicly Available
BMJ Open: first published as 10.1136/bmjopen-2018-027935 on 5 May 2019. Downloaded from BMJ Open is committed to open peer review. As part of this commitment we make the peer review history of every article we publish publicly available. When an article is published we post the peer reviewers’ comments and the authors’ responses online. We also post the versions of the paper that were used during peer review. These are the versions that the peer review comments apply to. The versions of the paper that follow are the versions that were submitted during the peer review process. They are not the versions of record or the final published versions. They should not be cited or distributed as the published version of this manuscript. BMJ Open is an open access journal and the full, final, typeset and author-corrected version of record of the manuscript is available on our site with no access controls, subscription charges or pay-per-view fees (http://bmjopen.bmj.com). If you have any questions on BMJ Open’s open peer review process please email [email protected] http://bmjopen.bmj.com/ on September 26, 2021 by guest. Protected copyright. BMJ Open BMJ Open: first published as 10.1136/bmjopen-2018-027935 on 5 May 2019. Downloaded from Treatment of stable chronic obstructive pulmonary disease: a protocol for a systematic review and evidence map Journal: BMJ Open ManuscriptFor ID peerbmjopen-2018-027935 review only Article Type: Protocol Date Submitted by the 15-Nov-2018 Author: Complete List of Authors: Dobler, Claudia; Mayo Clinic, Evidence-Based Practice Center, Robert D. -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2011) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2011) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

Scalable Green Chemistry Practices
Scalable Green Chemistry Practices Rakeshwar Bandichhor Ph.D. Director, API-R&D, Innovation Plaza IPDO, Dr. Reddy’s Laboratories, Hyderabad India ICGW-2013 6-8 December, 2013 Renaissance Hotel Mumbai, India http://www.drreddys.com/products/green-chemistry.html (Green Chemistry Website) Nature Medicine 2013, 19, 1200-1203 (Finding Right Chemistry) 1 Life Research Hope Significance of Chemistry •Whatever you hear, see, smell, taste, and touch involves chemistry and chemicals (matter). •And all these processes involve intricate series of chemical reactions and interactions in the biological system. • With such an enormous range of biological actions which are governed by chemistry therefore it is essential to know about this subject at some level in order to understand the world around us. Green Tea Coffee Cigarette >200 >1000 >7000 Chemicals 2 Life Research Hope Significance of Chemistry Chemistry Signifies Love and Hate Relationship -can’t live without it but can’t accept everything that it has. 3 Life Research Hope Green Chemistry •Evolving discipline it does mean that the definition of green may change tomorrow e.g. Grignard reaction was considered to be one of the best reactions but today it is being replaced with greener metal catalyzed transformations. •It is a subject that deals prevention of waste in any activity around us by design. 4 Life Research Hope Industry Sectors Energy alternatives Textile, printing, agro and construction Electronics and semiconductors Pharmaceutical, medical and biotech Cosmetics Retail and everyday commodity -

2021 Equine Prohibited Substances List
2021 Equine Prohibited Substances List . Prohibited Substances include any other substance with a similar chemical structure or similar biological effect(s). Prohibited Substances that are identified as Specified Substances in the List below should not in any way be considered less important or less dangerous than other Prohibited Substances. Rather, they are simply substances which are more likely to have been ingested by Horses for a purpose other than the enhancement of sport performance, for example, through a contaminated food substance. LISTED AS SUBSTANCE ACTIVITY BANNED 1-androsterone Anabolic BANNED 3β-Hydroxy-5α-androstan-17-one Anabolic BANNED 4-chlorometatandienone Anabolic BANNED 5α-Androst-2-ene-17one Anabolic BANNED 5α-Androstane-3α, 17α-diol Anabolic BANNED 5α-Androstane-3α, 17β-diol Anabolic BANNED 5α-Androstane-3β, 17α-diol Anabolic BANNED 5α-Androstane-3β, 17β-diol Anabolic BANNED 5β-Androstane-3α, 17β-diol Anabolic BANNED 7α-Hydroxy-DHEA Anabolic BANNED 7β-Hydroxy-DHEA Anabolic BANNED 7-Keto-DHEA Anabolic CONTROLLED 17-Alpha-Hydroxy Progesterone Hormone FEMALES BANNED 17-Alpha-Hydroxy Progesterone Anabolic MALES BANNED 19-Norandrosterone Anabolic BANNED 19-Noretiocholanolone Anabolic BANNED 20-Hydroxyecdysone Anabolic BANNED Δ1-Testosterone Anabolic BANNED Acebutolol Beta blocker BANNED Acefylline Bronchodilator BANNED Acemetacin Non-steroidal anti-inflammatory drug BANNED Acenocoumarol Anticoagulant CONTROLLED Acepromazine Sedative BANNED Acetanilid Analgesic/antipyretic CONTROLLED Acetazolamide Carbonic Anhydrase Inhibitor BANNED Acetohexamide Pancreatic stimulant CONTROLLED Acetominophen (Paracetamol) Analgesic BANNED Acetophenazine Antipsychotic BANNED Acetophenetidin (Phenacetin) Analgesic BANNED Acetylmorphine Narcotic BANNED Adinazolam Anxiolytic BANNED Adiphenine Antispasmodic BANNED Adrafinil Stimulant 1 December 2020, Lausanne, Switzerland 2021 Equine Prohibited Substances List . Prohibited Substances include any other substance with a similar chemical structure or similar biological effect(s). -
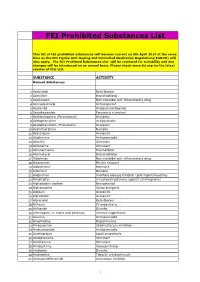
FEI Prohibited Substances List
FEI Prohibited Substances List This list of FEI prohibited substances will become current on 5th April 2010 at the same time as the FEI Equine Anti-Doping and Controlled Medication Regulations(EADCM) will also apply. The FEI Prohibted Substances List will be reviewed for suitability and any changes will be introduced on an annual basis. Please check www.fei.org for the latest version of this List. SUBSTANCE ACTIVITY Banned Substances 1 Acebutolol Beta blocker 2 Acefylline Bronchodilator 3 Acemetacin Non-steroidal anti-inflammatory drug 4 Acenocoumarol Anticoagulant 5 Acetanilid Analgesic/antipyretic 6 Acetohexamide Pancreatic stimulant 7 Acetominophen (Paracetamol) Analgesic 8 Acetophenazine Antipsychotic 9 Acetophenetidin (Phenacetin) Analgesic 10 Acetylmorphine Narcotic 11 Adinazolam Anxiolytic 12 Adiphenine Antispasmodic 13 Adrafinil Stimulant 14 Adrenaline Stimulant 15 Adrenochrome Haemostatic 16 Aformoterol Bronchodilator 17 Alclofenac Non-steroidal anti-inflammatory drug 18 Alcuronium Muscle relaxant 19 Aldosterone Hormone 20 Alfentanil Narcotic 21 Allopurinol Xanthine oxidase inhibitor (anti-hyperuricaemia) 22 Almotriptan 5-hydroxatryptamine agonist (antimigraine) 23 Alphadolone acetate Neurosteriod 24 Alphaprodine Opiod analgesic 25 Alpidem Anxiolytic 26 Alprazolam Anxiolytic 27 Alprenolol Beta blocker 28 Althesin IV anaesthetic 29 Althiazide Diuretic 30 Altrenogest (in males and geldings) Oestrus suppression 31 Alverine Antispasmodic 32 Amantadine Dopaminergic 33 Ambenonium Cholinesterase inhibition 34 Ambucetamide Antispasmodic 35 Amethocaine Local anaesthetic 36 Amfepramone Stimulant 37 Amfetaminil Stimulant 38 Amidephrine Vasoconstrictor 39 Amiloride Diuretic 40 Amineptine Tricyclic antidepressant 41 Aminoglutehthamide Aromatase inhibitor 1 FEI Prohibited Substances List This list of FEI prohibited substances will become current on 5th April 2010 at the same time as the FEI Equine Anti-Doping and Controlled Medication Regulations(EADCM) will also apply.