A Preliminary Study on the Toxic Combustion Products Testing of Polymers Used in High-Pressure Oxygen Systems
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Properties of Polypropylene Yarns with a Polytetrafluoroethylene
coatings Article Properties of Polypropylene Yarns with a Polytetrafluoroethylene Coating Containing Stabilized Magnetite Particles Natalia Prorokova 1,2,* and Svetlana Vavilova 1 1 G.A. Krestov Institute of Solution Chemistry of the Russian Academy of Sciences, Akademicheskaya St. 1, 153045 Ivanovo, Russia; [email protected] 2 Department of Natural Sciences and Technosphere Safety, Ivanovo State Polytechnic University, Sheremetevsky Ave. 21, 153000 Ivanovo, Russia * Correspondence: [email protected] Abstract: This paper describes an original method for forming a stable coating on a polypropylene yarn. The use of this method provides this yarn with barrier antimicrobial properties, reducing its electrical resistance, increasing its strength, and achieving extremely high chemical resistance, similar to that of fluoropolymer yarns. The method is applied at the melt-spinning stage of polypropylene yarns. It is based on forming an ultrathin, continuous, and uniform coating on the surface of each of the yarn filaments. The coating is formed from polytetrafluoroethylene doped with magnetite nanoparticles stabilized with sodium stearate. The paper presents the results of a study of the effects of such an ultrathin polytetrafluoroethylene coating containing stabilized magnetite particles on the mechanical and electrophysical characteristics of the polypropylene yarn and its barrier antimicrobial properties. It also evaluates the chemical resistance of the polypropylene yarn with a coating based on polytetrafluoroethylene doped with magnetite nanoparticles. Citation: Prorokova, N.; Vavilova, S. Properties of Polypropylene Yarns Keywords: coatings; polypropylene yarn; polytetrafluoroethylene; magnetite nanoparticles; barrier with a Polytetrafluoroethylene antimicrobial properties; surface electrical resistance; chemical resistance; tensile strength Coating Containing Stabilized Magnetite Particles. Coatings 2021, 11, 830. https://doi.org/10.3390/ coatings11070830 1. -

Tape-Unyte High Density Ptfe Tape
TAPE-UNYTE® HIGH DENSITY PTFE TAPE T-TAPE-SPEC PRODUCT DESCRIPTION COLOR/CONSISTENCY TENSILE STRENGTH - LONGITUDINAL 3000 PSI max - ASTM D882 (mod) PRODUCT The tape is white in color. It shall be free of visible voids, cracks, folds, ELONGATION TAPE-UNYTE® High Density PTFE Tape contamination and has consistent physical properties. TAPE-UNYTE® has an 50% min - ASTM D882 (mod) TYPE indefinite shelf life. THICKNESS ® TAPE-UNYTE is a heavy duty, all TEMPERATURE RANGE USE purpose, non-seizing, PTFE thread sealing .0040 ± .0005 Inches - ASTM D374-42 -A compound in tape form that produces a Gases: PACKAGING leakproof seal on all types of metal and -450EF (-268EC) to 500EF (260EC) plastic threaded connections. TAPE- UNYTE® is a high density, 4 MIL PTFE Liquids: U.S. Measure: (polytetrafluoroethylene) Tape supplied on -450EF (-268EC) to 500EF (260EC) finished spools. Stock Code Size PRESSURE RANGE USE F520 ¼" x 520" (.63 cm x 13.2 m) RECOMMENDED USES T260 ½" x 260" (1.27 cm x 6.6 m) Gases: T520 ½” x 520" (1.27 cm x 13.2 m) TAPE-UNYTE® will not transfer any taste up to 10,000 PSI (1450 kPa) T1296 ½” x 1296" (1.27 cm x 32.9 m) or odor to the system being sealed. Liquids: W260 ¾" x 260" (1.9 cm x 6.6 m) Excellent for food and water systems. up to 3,000 PSI (435 kPa) W520 ¾" x 520" (1.9 cm x 13.2 m) TAPE-UNYTE® will never harden, and is an X260 1" x 260" (1.9 cm x 6.6 m) anti-galling tape making it possible to The system may be pressurized disassemble pipes and bolts easily after immediately after assembly. -

DUPONT DATA BOOK SCIENCE-BASED SOLUTIONS Dupont Investor Relations Contents 1 Dupont Overview
DUPONT DATA BOOK SCIENCE-BASED SOLUTIONS DuPont Investor Relations Contents 1 DuPont Overview 2 Corporate Financial Data Consolidated Income Statements Greg Friedman Tim Johnson Jennifer Driscoll Consolidated Balance Sheets Vice President Director Director Consolidated Statements of Cash Flows (302) 999-5504 (515) 535-2177 (302) 999-5510 6 DuPont Science & Technology 8 Business Segments Agriculture Electronics & Communications Industrial Biosciences Nutrition & Health Performance Materials Ann Giancristoforo Pat Esham Manager Specialist Safety & Protection (302) 999-5511 (302) 999-5513 20 Corporate Financial Data Segment Information The DuPont Data Book has been prepared to assist financial analysts, portfolio managers and others in Selected Additional Data understanding and evaluating the company. This book presents graphics, tabular and other statistical data about the consolidated company and its business segments. Inside Back Cover Forward-Looking Statements Board of Directors and This Data Book contains forward-looking statements which may be identified by their use of words like “plans,” “expects,” “will,” “believes,” “intends,” “estimates,” “anticipates” or other words of similar meaning. All DuPont Senior Leadership statements that address expectations or projections about the future, including statements about the company’s strategy for growth, product development, regulatory approval, market position, anticipated benefits of recent acquisitions, timing of anticipated benefits from restructuring actions, outcome of contingencies, such as litigation and environmental matters, expenditures and financial results, are forward looking statements. Forward-looking statements are not guarantees of future performance and are based on certain assumptions and expectations of future events which may not be realized. Forward-looking statements also involve risks and uncertainties, many of which are beyond the company’s control. -

Trade Names and Manufacturers
Appendix I Trade names and manufacturers In this appendix, some trade names of various polymeric materials are listed. The list is intended to cover the better known names but it is by no means exhaustive. It should be noted that the names given may or may not be registered. Trade name Polymer Manufacturer Abson ABS polymers B.F. Goodrich Chemical Co. Acrilan Polyacrylonitrile Chemstrand Corp. Acrylite Poly(methyl methacrylate) American Cyanamid Co. Adiprene Polyurethanes E.I. du Pont de Nemours & Co. Afcoryl ABS polymers Pechiney-Saint-Gobain Alathon Polyethylene E.I. du Pont de Nemours & Co. Alkathene Polyethylene Imperial Chemical Industries Ltd. Alloprene Chlorinated natural rubber Imperial Chemical Industries Ltd. Ameripol cis-1 ,4-Polyisoprene B.F. Goodrich Chemical Co. Araldite Epoxy resins Ciba (A.R.L.) Ltd. Arnel Cellulose triacetate Celanese Corp. Arnite Poly(ethylene terephthalate) Algemene Kunstzijde Unie N.Y. Baypren Polychloroprene Farbenfabriken Bayer AG Beetle Urea-formaldehyde resins British Industrial Plastics Ltd. Ben vic Poly(vinyl chloride) Solvay & Cie S.A. Bexphane Polypropylene Bakelite Xylonite Ltd. Butacite Poly( vinyl butyral) E.I. du Pont de Nemours & Co. Butakon Butadiene copolymers Imperial Chemical Industries Ltd. Butaprene Styrene-butadiene copolymers Firestone Tire and Rubber Co. Butvar Poly(vinyl butyral) Shawinigan Resins Corp. Cap ran Nylon 6 Allied Chemical Corp. Carbowax Poly(ethylene oxide) Union Carbide Corp. Cariflex I cis-1 ,4-Polyisoprene Shell Chemical Co. Ltd. Carina Poly(vinyl chloride) Shell Chemical Co. Ltd. TRADE NAMES AND MANUFACTURERS 457 Trade name Polymer Manufacturer Carin ex Polystyrene Shell Chemical Co. Ltd. Celcon Formaldehyde copolymer Celanese Plastics Co. Cellosize Hydroxyethylcellulose Union Carbide Corp. -

United States Patent (19) 11 Patent Number: 4,481,333 Fleischer Et Al
United States Patent (19) 11 Patent Number: 4,481,333 Fleischer et al. 45 Date of Patent: Nov. 6, 1984 54 THERMOPLASTIC COMPOSITIONS 58 Field of Search ................................ 525/192, 199 COMPRISING WINYL CHLORIDE POLYMER, CLPE AND FLUOROPOLYMER 56) References Cited U.S. PATENT DOCUMENTS 75) Inventors: Dietrich Fleischer, Darmstadt; Eckhard Weber, Liederbach; 3,005,795 10/1961 Busse et al. ......................... 525/199 3,294,871 2/1966 Schmitt et al. ...... 52.5/154 X Johannes Brandrup, Wiesbaden, all 3,299,182 1/1967 Jennings et al. ... ... 525/192 of Fed. Rep. of Germany 3,334,157 8/1967 Larsen ..................... ... 525/99 73 Assignee: Hoechst Aktiengesellschaft, Fed. 3,940,456 2/1976 Fey et al. ............................ 525/192 Rep. of Germany Primary Examiner-Carman J. Seccuro (21) Appl. No.: 566,207 Attorney, Agent, or Firm-Connolly & Hutz 22 Filed: Dec. 28, 1983 57 ABSTRACT 30 Foreign Application Priority Data The invention relates to a thermoplastic composition which comprises vinyl chloride polymers and chlori Dec. 31, 1982 (DE Fed. Rep. of Germany ....... 3248.731 nated polyethylene and which contains finely divided 51) Int. Cl. ...................... C08L 23/28; C08L 27/06; fluoropolymers and has a markedly improved process C08L 27/18 ability, particularly when shaped by extrusion. 52 U.S. C. .................................... 525/192; 525/199; 525/239 7 Claims, No Drawings 4,481,333 2 iaries and do not provide a solution to the present prob THERMOPLASTC COMPOSITIONS lem. COMPRISINGVINYL CHLORIDE POLYMER, -

Study of Linear Low Density Polyethylene/Polytetrafluoroethylene-G-1,3-Butadiene Processability
Study of linear low density polyethylene/polytetrafluoroethylene-g-1,3-butadiene processability H. F. R. Ferreto; L. F. C. P. Lima; D. F. Parra; A. B. Lugão Instituto de Pesquisas Energéticas e Nucleares - IPEN Av. Lineu Prestes, 2242, 05508-900 Butantã, São Paulo, SP. E-mail: [email protected] Abstract The extrusion of linear low density polyethylene (LLDPE) is limited by a process related defect known as ‘melt fracture’ or ‘sharkskin’, which is a surface defect of the extruded polymer. This defect results in a product with a rough surface that lacks luster and in alterations of specific surface properties. The aim of this study was to obtain a recycled polytetrafluoroethylene polymer with an olefin that could improve the extrudability of the LLDPE. The copolymer was obtained by irradiating recycled PTFE in an inert atmosphere followed by the addition of an olefinic monomer to graft the latter in the polymeric matrix (PTFE). The olefinic monomer used was 1,3-butadiene. The specimens were studied using Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA) and differential thermogravimetry (DTG). Therefore, in an effort to enhance the processability and so the drawability, it has been found helpful to add a small amount of copolymer. 0.2-2.0 wt% of the copolymer that was obtained was mixed with LLDPE. The rheological properties of the mixture were determined with, a torque Haake rheometer, a rotational Physica rheometer. The results indicated that the process used rendered a copolymer which when added to LLDPE, improved the extrusion process and eliminated the defect ‘melt fracture’. Keywords: gamma radiation, PTFE, LLDPE, grafting 1 Introduction The radiation-induced grafting for modification of properties from commercial polymer is a largely studied technique. -

Microstructure and Properties of Polytetrafluoroethylene Composites
coatings Article Microstructure and Properties of Polytetrafluoroethylene Composites Modified by Carbon Materials and Aramid Fibers Fubao Zhang *, Jiaqiao Zhang , Yu Zhu, Xingxing Wang and Yuyang Jin School of Mechanical Engineering, Nantong University, Nantong 226019, China; [email protected] (J.Z.); [email protected] (Y.Z.); [email protected] (X.W.); [email protected] (Y.J.) * Correspondence: [email protected]; Tel.: +86-13646288919 Received: 12 October 2020; Accepted: 16 November 2020; Published: 18 November 2020 Abstract: Polytetrafluoroethylene (PTFE) is polymerized by tetrafluoroethylene, which has high corrosion resistance, self-lubrication and high temperature resistance. However, due to the large expansion coefficient, high temperature will gradually weaken the intermolecular bonding force of PTFE, which will lead to the enhancement of permeation absorption and the limitation of the application range of fluoroplastics. In order to improve the performance of PTFE, the modified polytetrafluoroethylene, filled by carbon materials and aramid fiber with different scales, is prepared through the compression and sintering. Moreover, the mechanical properties and wear resistance of the prepared composite materials are tested. In addition, the influence of different types of filler materials and contents on the properties of PTFE is studied. According to the experiment results, the addition of carbon fibers with different scales reduces the tensile and impact properties of the composite materials, but the elastic modulus and wear resistance are significantly improved. Among them, the wear rate of 7 µm carbon fiber modified PTFE has decreased by 70%, and the elastic modulus has increased by 70%. The addition of aramid fiber filler significantly reduces the tensile and impact properties of the composite, but its elastic modulus and wear resistance are significantly improved. -

Information on Plastics
Plastics By K. P. Shah Email: kpshah123[at]gmail.com (Please replace [at] with @) Committed to improve the Quality of Life The information contained in this article represents a significant collection of technical information on plastics. This information will help to achieve increased reliability at a decreased cost. Assemblage of this information will provide a single point of reference that might otherwise be time consuming to obtain. Most of information given in this article is mainly derived from the literature on the subject indicated in the reference given at the end of this article. For more information, please refer it. All information contained in this article has been assembled with great care. However, the information is given for guidance purposes only. The ultimate responsibility for its use and any subsequent liability rests with the end user. Please see the disclaimer uploaded on http://www.practicalmaintenance.net. (Edition: April 2013) 1 Plastics www.practicalmaintenance.net Plastics Plastics are used in many products and components like compression packings, gaskets, seals, bearings, liners, etc. Instead of giving detail information about them in articles about these products, useful information about them is given in this article. Information about plastics and commonly used plastics like HDPE, UHMWPE, PTFE and nylon is given in this article. What is Plastic? Plastic is the general common term for a wide range of synthetic or semi-synthetic materials used in a huge, and growing, range of applications. All plastics were soft and moldable during their production - that's why they're called plastics. The Greek word plasticós means "to mold." Plastics are organic, the same as wood, paper or wool. -

Microwave Dielectric Properties of Glass-Reinforced Polymers
http://www.e-polymers.org e-Polymers 2005, no. 004. ISSN 1618-7229 Short communication: Microwave dielectric properties of glass-reinforced polymers Luís Cadillon Costa 1 *, Susana Devesa 1, Paulo André 1,2 1 Physics Department, University of Aveiro, 3810-193 Aveiro, Portugal; [email protected] 2 Telecommunications Institute, 3810-193 Aveiro, Portugal; [email protected] (Received: October 15, 2004; published: January 21, 2005) This work has been presented at the 12th Annual POLYCHAR World Forum on Advanced Materials, January 6-9, 2004, in Guimaraes, Portugal Abstract: The well-balanced combination of properties of some polymers makes them good materials for industrial microwave applications, such as telecommu- nications or microwave ovens. Particular electrical properties are usually needed and can be controlled by additives. In this work, we present the results of complex dielectric permittivity measurements, ε* = ε’ - iε’’, in the microwave frequency region (2.7 and 12.8 GHz), at constant temperature 300 K, on different glass-reinforced and pigmented plastics (poly(butylene terephthalate), polypropylene and acrylo- nitrile-butadiene-styrene), using the resonant cavity method. We measured the shift in the resonant frequency of the cavity, ∆f, caused by the insertion of the sample, which can be related to the real part of complex permittivity, ε’, while the change in the inverse of the quality factor of the cavity, ∆(1/Q), gives the imaginary part, ε’’. 1. Introduction A very versatile family of thermoplastics includes acrylonitrile-butadiene-styrene (ABS), polypropylene (PP) and poly(butylene terephthalate) (PBT). They present interesting properties, as low thermal expansion coefficient, high dielectric strength, and very low dielectric losses, and have good resistance to chemical attack [1-3]. -

Dupont™ Zytel® Product Guide and Properties
DuPont™ Zytel® nylon resin Product guide and properties 1 2 4 3 ® Registered trademarks of E.I. du Pont de Nemours and Company The miracles of science™ is a trademark of E.I. du Pont de Nemours and Company ST801 16 Toughness-Stiffness Ratio of various ZYTEL® resins compared to ZYTEL® 101L 14 490 12 10 Toughened ZYTEL® 1) ® Toughened glass reinforced ZYTEL ® Toughness 8 Glass reinforced ZYTEL 6 408 80G33 4 450 80G25 70G43 80G14 114 70G35 70G503) 70G30 2 79G13 42 101 70G25 70G20 151 135 0 246 8 101214 Stiffness2) 1) Notched Izod impact, DAM 2) Flexural modulus, 50% RH 3) Preliminary data 5 Photographs 1 – Residual circuit breaker – glass-mineral reinforced 2 – Air intake manifold – glass reinforced 3 – Sole for cycling shoes – glass reinforced 6 4 – Flat filter housing – glass reinforced 5 – Resonator – glass reinforced 6 – Hedge-trimmer housing – glass reinforced 2 DuPont™ Zytel® nylon resin Properties of ZYTEL® HTN resins are given in the bro- chure “ZYTEL® HTN – Product guide and properties”. Introduction Mineral and mineral/glass reinforced nylons are also ZYTEL® is DuPont’s registered trademark for its com- available under the MINLON® trademark. Information prehensive range of nylon resins. Since the invention on these products is given in the brochure “MINLON® – of nylon by DuPont in the 1930s, it has become the Product guide and properties”. most widely used of all engineering polymers. Due to their excellent balance of properties, nylon components Data (produced by injection moulding, extrusion or blow All data in this brochure is taken from Campus version moulding) find extensive use in many applications 4.0 (measured according to ISO standards), except including: automotive, electrical/electronic, domestic where otherwise specified. -

Candidats Potentiels
Polymères possédant un bon potentiel afin de répondre à l’application de RDDC - Valcartier Contrat: W7701 – 4501411762 Titre: Sélection de polymères Dr. AutoritéFrédéric Byette technique: Emmanuela Diaz, Scientifique de la Défense Prof. Christian Pellerin Université de Montréal, Juillet 2016 DRDC-RDDC-2017-C020 Table des matières Fluorinated ethylene propylene copolymer / FEP (p.3) Perfluoroalkoxy alkane / PFA (p.6) Poly(ethene-co-tetrafluoroethylene) / ETFE (p.9) Poly(tetrafluoroethylene) / PTFE (p.12) Poly(vinylidene fluoride) / PVdF (p.15) Polybutylene terephthalate / PBT (p.17) Polyethylene terephthalate / PET (p.20) Polytrimethylene terephthalate / PTT (p.25) Polyphenylene sulfide / PPS (p.27) Polycarbonate / PC (p.29) Polyoxymethylene / POM (p.32) Polypropylene isotactique / iPP (p.34) Polycaprolactone / PCL (p.37) Polypivalolactone / PPVL (p.38) Nylon 6 (p.40) Nylon 6,6 (p.42) Poly para aramid (p.44) 2 &! )+#2$-'*!&,, 1$&(*'(1$&'('$1%* %#&%&# #,'% 2 #2 '+,$'&<$'& G %'-*+E-('&,H #$%6 #!2+%!@*!+,$$!& %%,%#4=:E%?:E2-!G*6+!+,&, "-+)-B3@ORMY? !%!,'(6*,!'&&$$XNRM@OMMY<%X OSMYH #,#% 2&XN?PQQ ,$$%%*5&%*5&%,2-! $$#, 5%,#-%8;<::7B::4;6;<3?7B3=F92-! !%#42 Indice de Bande Coefficient réfraction (cm-1) d'absorption FEP 1.344 1147 0.535 P Spectres polarisés réflexion spéculaire (indice d’absorption k après transformation de Kramers-Kronig): Ratio d’étirement approximatif = 350% Spectres de réflectance avant et après incubation 24 h à T = 50°C suite à l’étirement: 4 Estimation du DOLP: Bande (cm-1) DOLP FEP 1147 -0.57 Notes et commentaires: Produit similaire au Teflon PTFE, cependant plus souple et donc plus facile à étirer. Très similaire au PFA. Le DOLP est peu affecté suite à l’incubation 24 h à 50°C. -
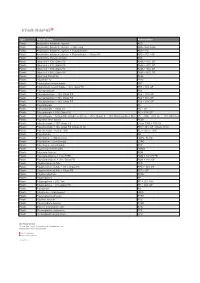
Type Material Name Abbreviation Plastic Acrylonitrile Butadiene
Type Material Name Abbreviation Plastic Acrylonitrile butadiene styrene ABS Plastic Acrylonitrile butadiene styrene - High-Temp ABS - high temp Plastic Acrylonitrile butadiene styrene + Polycarbonate ABS + PC Plastic Acrylonitrile butadiene styrene + Polycarbonate + Glass Fill ABS + PC + GF Plastic Acrylonitrile styrene acrylate ASA Plastic Nylon 6-6 + 10% Glass Fill PA66 + 10% GF Plastic Nylon 6-6 + 20% Glass Fill PA66 + 20% GF Plastic Nylon 6-6 + 30% Glass Fill PA66 + 30% GF Plastic Nylon 6-6 + 50% Glass Fill PA66 + 50% GF Plastic Nylon 6-6 Polyamide PA66 Plastic Polyamide 12 PA12 Plastic Polybutylene terephthalate PBT Plastic Polybutylene terephthalate + 30% Glass Fill PBT+ 30% GF Plastic Polycaprolactam PA6 Plastic Polycaprolactam + 20% Glass Fill PA6 + 20% GF Plastic Polycaprolactam + 30% Glass Fill PA6 + 30% GF Plastic Polycaprolactam + 50% Glass Fill PA6 + 50% GF Plastic Polycarbonate PC Plastic Polycarbonate + Glass Fill PC + GF Plastic Polycarbonate + 10% Glass Fill PC + 10% GF Plastic Polycarbonate + Acrylonitrile butadiene styrene + 20% Glass Fill + 10% Stainless Steel fiber PC + ABS + 20% GF + 10% SS Fiber Plastic Polyether ether ketone PEEK Plastic Polyetherimide + 30% Glass Fill Ultem 1000 + 30% GF Plastic Polyetherimide + 40% Glass Fill (Ultem 2410) PEI + 40% GF (Ultem 2410) Plastic Polyetherimide + Ultem 1000 PEI + Ultem 1000 Plastic Polyethylene PE Plastic Polyethylene - High-Density HDPE, PEHD Plastic Polyethylene - Low-Density LDPE Plastic Polyethylene terephthalate PET Plastic Polymethyl methacrylate PMMA Plastic Polyoxymethylene