Distribution of Amphipod Communities in the Middle to Upper Rhine and Five of Its Tributaries
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Die Nase (Chondrostoma Nasus) Im Einzugsgebiet Des Bodensees – Grundlagenbericht 1
Die Nase (Chondrostoma nasus) im Einzugsgebiet des Bodensees – Grundlagenbericht 1 Die Nase (Chondrostoma nasus) im Einzugsgebiet des Bodensees Grundlagenbericht für internationale Maßnahmenprogramme HYDRA Konstanz, Juni 2019 Internationale Bevollmächtigtenkonferenz für die Bodenseefischerei (IBKF) IBKF – Internationale Bevollmächtigtenkonferenz für die Bodenseefischerei 2 Die Nase (Chondrostoma nasus) im Einzugsgebiet des Bodensees – Grundlagenbericht Die Nase (Chondrostoma nasus) im Einzugsgebiet des Bodensees Grundlagenbericht für internationale Maßnahmenprogramme Autor: Peter Rey GIS: John Hesselschwerdt Recherchen: Johannes Ortlepp Andreas Becker Begleitung: IBKF – Arbeitsgrupppe Wanderfische: Mag. DI Roland Jehle, Amt für Umwelt, Liechtenstein (Vorsitz) Dr. Marcel Michel, Amt für Jagd und Fischerei, Graubünden Roman Kistler, Jagd- und Fischereiverwalter des Kantons Thurgau Dario Moser, Jagd- und Fischereiverwalter des Kantons Thurgau LR Dr. Michael Schubert, Bayerische Landesanstalt für Landwirtschaft – Institut für Fischerei ORR Dr. Roland Rösch, Ministerium für Ländlichen Raum und Verbraucherschutz Baden-Württemberg Dr. Dominik Thiel, Amt für Natur, Jagd und Fischerei des Kantons St. Gallen Michael Kugler, Amt für Natur, Jagd und Fischerei des Kantons St. Gallen Mag. Nikolaus Schotzko, Amt der Vorarlberger Landesregierung, Landesfischereizentrum Vorarlberg RegD. Dr. Manuel Konrad, Regierungspräsidium Tübingen, Fischereibehörde Uwe Dußling, Regierungspräsidium Tübingen, Fischereibehörde Juni 2019 Internationale Bevollmächtigtenkonferenz -

Anlage 4 Präsentation Von Ulrich Androsch
GEWÄSSERVERBAND Bergstraße An der Weschnitz 1 64653 Lorsch 0 62 51 / 5 24 85 [email protected] www.gewaesserverband-bergstrasse.de 2. Auftaktveranstaltung WRRL Kreis Bergstraße AK Oberflächengewässer (AK OW) Umfang im Verbandsgebiet Umsetzungsplanung Was ist bisher passiert Was passiert noch Seite 16 WRRL-Umsetzung im Kreis Bergstraße 1. WRRL-Gewässer Bergstraße WRRL-Umsetzung im Kreis Bergstraße 2. Wanderhindernisse, ~ 250 Stck. Bergstraße Seite 17 WRRL-Umsetzung im Kreis Bergstraße 3. Strukturdefizite, i. M. > 90 % Bergstraße Seite 18 Seite 19 Mitlechtern Mitlechtern Seite 20 WRRL-Umsetzung im Kreis Bergstraße 4. Bisherige Maßnahmenumsetzung Gernsheim Langwaden Zwingenberg Groß Rohrheim Biblis Lindenfels Einhausen Bensheim Lorsch Heppenheim Fürth Bürstadt Rimbach Mörlenbach Lampertheim umge in Vor- Strukturaufwertung Birkenau setzt bereitung Weinheim Beseitigung Wanderhindernis –umgesetzt4 Viernheim Beseitigung Wanderhindernis –in Vorbereitung Bergstraße Seite 21 WRRL-Umsetzung im Kreis Bergstraße 4. Bisherige Maßnahmenumsetzung Gernsheim Langwaden Zwingenberg Groß Rohrheim Biblis Lindenfels Einhausen Bensheim Lorsch Heppenheim Fürth Bürstadt Weschnitz: Längsdurchgängigkeit Weschnitz/NW bis Landesgrenze hergestellt (1 WH) Rimbach Längsdurchgängigkeit oberes Weschnitztal, 2 WH in Vorbereitung Längsdurchgängigkeit Weschnitz/AW noch in 2011 Strukturaufwertung Weschnitz: 3 km (2011), 7,5 km in Vorbereitung (RP, ab 2012) Meerbach: Mörlenbach Längsdurchgängigkeit bis oberhalb Bensheim hergestellt (2 WH) Lampertheim Strukturaufwertung: -

The Ahr and the Emergence of German Reds
©2010 Sommelier Journal. May not be distributed without permission. www.sommelierjournal.com The Ahr and the emergence of German reds CHRISTOPHER BATES, CWE t is not exactly breaking news that Germany to pass Müller-Thurgau to become the coun- has been making red wines able to stand try’s second-most-planted grape variety behind side by side with many of the world’s famous Riesling. While Müller-Thurgau production Ilabels. In 2006, a collector traded a bottle has declined since 1975, the percentage of Ger- of Domaine de la Romanée-Conti for a bottle of man vineyard land dedicated to Riesling has re- hans-Peter Wöhrwag’s 2003 Untertürkheimer mained incredibly stable at around 21%, while herzogenberg Pinot Noir from Württemberg. A the amount devoted to Spätburgunder has risen one-off, for sure, but it may also have been a hint from 3% to 12%. of things to come. In 2008, Decanter magazine Even though the current hype makes it easy named a German red wine the best in the world to think of Germany as a new red-wine-produc- for its variety, and again, it was a Pinot Noir: ing culture, red-grape plantings were document- Weingut Meyer-Näkel’s 2005 Spätburgunder ed here as early as 570 A.D., and Pinot Noir was Dernauer Pfarrwingert Grosses Gewächs. identified as early as 1318. It was not until 1435 Actually, nearly a third of German vine- that plantings of Riesling were first recorded. In yards are planted to red grapes. Spätburgunder, the Ahr, it is commonly believed that vines were as Pinot Noir is known in Germany, is about grown in Roman times, although the first docu- 56 January 31, 2010 Special Report Jean Stodden Recher Herr- enberg vineyard. -

EMS INFORMATION BULLETIN Nr 144
16/07/2021 EMSR517 – Flood in Western Germany EMSR518 – Flood in Belgium EMSR519 – Flood in Switzerland EMSR520 – Flood in The Netherlands EMS INFORMATION BULLETIN Nr 144 THE COPERNICUS EMERGENCY MANAGEMENT SERVICE The Copernicus Emergency Management Service forecasts, notifies, and monitors devastating floods in Germany, Netherlands, Belgium and Switzerland CEMS flood forecasting and notifying in Germany On 9 and 10 July, flood forecasts by the European Flood Awareness System (EFAS) of the Copernicus Emergency Management Service indicated a high probability of flooding for the Rhine River basin, affecting Switzerland and Germany. Subsequent forecasts also indicated a high risk of flooding for the Meuse River basin, affecting Belgium. The magnitude of the floods forecasted for the Rhine River basin increased significantly in this period. The first EFAS notifications were sent to the relevant national authorities starting on 10 July and, with the continuously updated forecasts, more than 25 notifications were sent for specific regions of the Rhine and Meuse River basins in the following days until 14 July. Figure: EFAS flood forecast from 12.07.2021 00:00 UTC Providing early and current maps of flooded areas On 13 July, the CEMS Rapid Mapping component was activated to map the ongoing floods in parts of Western Germany (EMSR517 Mapping Website , EMSR517 Activation Viewer). As a flood peak was foreseen on 16 July for segments of other rivers, CEMS preemptively acquired satellite images of the vulnerable area through Pre-Tasking on 14 July. These early images informed ensuing activations by the CEMS Rapid Mapping component based on the EFAS forecasts for areas in Belgium, Netherlands, Germany, Switzerland and France. -

Pham Thi Minh Thu
Institut für Wasserwirtschaft und Kulturtechnik Universität Karlsruhe (TH) A Hydrodynamic-Numerical Model of the River Rhine Pham Thi Minh Thu Heft 213 Mitteilungen des Instituts für Wasserwirtschaft und Kulturtechnik der Universität Karlsruhe (TH) mit ″Theodor-Rehbock-Wasserbaulaboratorium″ Herausgeber: Prof. Dr.-Ing. Dr. h. c. Franz Nestmann, Ordinarius 2002 A Hydrodynamic-Numerical Model of the River Rhine Zur Erlangung des akademischen Grades eines DOKTOR-INGENIEURS der Fakultät für Bauingenieur- und Vermessungswesen der Universität Fridericiana zu Karlsruhe (TH) genehmigte DISSERTATION von Dipl. -Ing. Pham Thi Minh Thu aus Hanoi, Vietnam Tag der mündlichen Prüfung: 13. Februar 2002 Hauptreferent: Prof. Dr.-Ing. Dr. h.c. mult. Franz Nestmann 1. Korreferent: Prof. Dr.-Ing. Helmut Scheuerlein 2. Korreferent: Prof. Dr.-Ing. habil. Hans Helmut Bernhart Karlsruhe, 2002 Vorwort Der Rhein unterliegt seit Jahrhunderten anthropogenen Eingriffen, die sich auf das Ablaufverhalten von Hochwasserwellen auswirken. Der Schutz und die Wiederherstellung ökologisch funktionsfähiger, naturnaher Gewässer ebenso wie eine bessere Hochwasserregulierung sind wesentliche Aufgaben der Wasserwirtschaft, wobei eine gesamtheitliche Betrachtungsweise erforderlich ist. Um die hydraulischen Auswirkungen einer Rückgewinnung von Retentionsräumen auf Hochwasserereignisse zu quantifizieren, wurde von Frau Dr. Minh Thu in dieser Forschungsarbeit ein hydrodynamisch-numerisches Modell für die gesamte deutsche Teilstrecke des freifließenden Rheins erstellt. Es besteht aus -

Berlin, 18. August 2021 Extent of Damage on the Night from 14Th To
Berlin, 18. August 2021 Extent of damage On the night from 14th to 15th July, heavy rain resulted in quickly rising water levels of a number of smaller rivers, e.g., the rivers Ahr and Erft, in the federal states of Rheinland-Pfalz and Nordrhein-Westphalen. Many villages and smaller towns situated at these rivers experienced flooding which surpassed the expected extreme high-water events (likelihood of once in 100-200 years). The floods caused massive damages to buildings, streets, but also vegetation alongside these rivers. In addition, many more houses experienced flooding of the basement and ground floor. Only for the Ahr valley alone, it is estimated that 17,000 people have lost their homes; 133 people have died and 766 were hurt (as reported by SWR on 13 August 20211). Since the catastrophe response is organised on the municipal and federal level, it will take more time to arrive at general numbers encompassing all the districts in both federal states. With regard to damages to cultural property, the extent is still unclear as not one organisation or official institution is tasked to gather this information, including Blue Shield. We can say that the full spectrum of cultural property has been hit: archives, museums, libraries and monuments & sites (including churches). The last group is expected to show the worst damage as they could not be evacuated and are more susceptible to the second wave of damage, i.e., through demolitions or simply bad repairs. With this many livelihoods having been lost and insurance companies being more interested in saving costs (building new houses), privately owned monuments face a high risk of disappearing. -

Stadtplan Fürth
a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a aa a a a a a a a aaa a a a a a aa a a a a a a a a a a a aa a a a a a a a a a a aa a a aa a a a a a a aa a a a aa a a a a a aa a a a a a a a a a a a a a a a a a a a a a a a a a a a a a aa a a a aa asb a tutz ach erg S a a a aa a a a aa a a a a D a a a a a a a a a aa a a aa a a a a a a aaa a a aaaa aaaaa a a a a a nacha a an a Glashüt- Krehberg- anach Winterkasten aa a a a a a a a a a a a a a a a a a W Ð 1 a aa Schannen- nach Winkel nach Reichelsheim a a aaa a i a nach Kolmbach aaa tenweg e r F a 494,6 sens e ch - aa bach tr. -
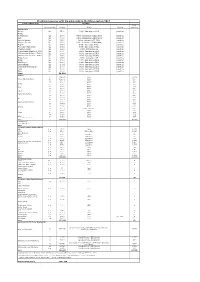
Stocking Measures with Big Salmonids in the Rhine System 2017
Stocking measures with big salmonids in the Rhine system 2017 Country/Water body Stocking smolt Kind and stage Number Origin Marking equivalent Switzerland Wiese Lp 3500 Petite Camargue B1K3 genetics Rhine Riehenteich Lp 1.000 Petite Camargue K1K2K4K4a genetics Birs Lp 4.000 Petite Camargue K1K2K4K4a genetics Arisdörferbach Lp 1.500 Petite Camargue F1 Wild genetics Hintere Frenke Lp 2.500 Petite Camargue K1K2K4K4a genetics Ergolz Lp 3.500 Petite Camargue K7C1 genetics Fluebach Harbotswil Lp 1.300 Petite Camargue K7C1 genetics Magdenerbach Lp 3.900 Petite Camargue K5 genetics Möhlinbach (Bachtele, Möhlin) Lp 600 Petite Camargue B7B8 genetics Möhlinbach (Möhlin / Zeiningen) Lp 2.000 Petite Camargue B7B8 genetics Möhlinbach (Zuzgen, Hellikon) Lp 3.500 Petite Camargue B7B8 genetics Etzgerbach Lp 4.500 Petite Camargue K5 genetics Rhine Lp 1.000 Petite Camargue B2K6 genetics Old Rhine Lp 2.500 Petite Camargue B2K6 genetics Bachtalbach Lp 1.000 Petite Camargue B2K6 genetics Inland canal Klingnau Lp 1.000 Petite Camargue B2K6 genetics Surb Lp 1.000 Petite Camargue B2K6 genetics Bünz Lp 1.000 Petite Camargue B2K6 genetics Sum 39.300 France L0 269.147 Allier 13457 Rhein (Alt-/Restrhein) L0 142.000 Rhine 7100 La 31.500 Rhine 3150 L0 5.000 Rhine 250 Doller La 21.900 Rhine 2190 L0 2.500 Rhine 125 Thur La 12.000 Rhine 1200 L0 2.500 Rhine 125 Lauch La 5.000 Rhine 500 Fecht und Zuflüsse L0 10.000 Rhine 500 La 39.000 Rhine 3900 L0 4.200 Rhine 210 Ill La 17.500 Rhine 1750 Giessen und Zuflüsse L0 10.000 Rhine 500 La 28.472 Rhine 2847 L0 10.500 Rhine 525 -

Rapid Attribution of Heavy Rainfall Events Leading to the Severe Flooding in Western Europe During July 2021
Rapid attribution of heavy rainfall events leading to the severe flooding in Western Europe during July 2021 Contributors Frank Kreienkamp1, Sjoukje Y. Philip2, Jordis S. Tradowsky1,4, Sarah F. Kew2, Philip Lorenz1, Julie Arrighi7,8,9, Alexandre Belleflamme16, Thomas Bettmann18, Steven Caluwaerts13,19, Steven C. Chan14, Andrew Ciavarella22, Lesley De Cruz13, Hylke de Vries2, Norbert Demuth18, Andrew Ferrone17, Erich M. Fischer6, Hayley J. Fowler14, Klaus Goergen16, Dorothy Heinrich7, Yvonne Henrichs18, Geert Lenderink2, Frank Kaspar10, Enno Nilson15, Friederike E L Otto11, Francesco Ragone13,20, Sonia I. Seneviratne6, Roop K. Singh7, Amalie Skålevåg, Piet Termonia13,19, Lisa Thalheimer11, Maarten van Aalst7,8,21, Joris Van den Bergh13, Hans Van de Vyver13, Stéphane Vannitsem13, Geert Jan van Oldenborgh2,3, Bert Van Schaeybroeck13, Robert Vautard5, Demi Vonk8, Niko Wanders12 1 - Deutscher Wetterdienst (DWD), Regionales Klimabüro Potsdam, Potsdam, Germany; 2 - Royal Netherlands Meteorological Institute (KNMI), De Bilt, The Netherlands; 3 - Atmospheric, Oceanic and Planetary Physics, University of Oxford, UK; 4 - Bodeker Scientific, Alexandra, New Zealand; 5 - Institut Pierre-Simon Laplace, CNRS, Paris, France; 6 - Institute for Atmospheric and Climate Science, Department of Environmental Systems Science, ETH Zurich, Zurich, Switzerland; 7 - Red Cross Red Crescent Climate Centre, The Hague, the Netherlands; 8 - Faculty of Geo-Information Science and Earth Observation (ITC), University of Twente, Enschede, the Netherlands; 9 - Global Disaster -

Vineyard Soils of Rhineland-Palatinate
Vineyard Soils of Rhineland-Palatinate Rocks. Soils. Terroir. Impressum Publishers: Foreword Ministerium für Wirtschaft, Klimaschutz, Energie und Landesplanung Rheinland-Pfalz Ministry for Economic Affairs, Climate Protection, Energy and Spatial Planning Rhineland-Palatinate Dear ladies and gentlemen, Stiftsstraße 9, 55116 Mainz for ten years now, the “Soil of the Year” for the upcoming year is announced on December 5th, [email protected] the International World Soil Day. When the vineyard soil was selected for 2014, the federal www.mwkel.rlp.de state of Rhineland-Palatinate, as the largest wine-growing state in Germany gladly assumed patronage for this soil. The brochure “Vineyard Soils of Rhineland-Palatinate“ introduces the Ministerium für Umwelt, Landwirtschaft, Ernährung, Weinbau und Forsten Rheinland-Pfalz large diversity of soils of the wine-growing areas Ahr, Mittelrhein, Mosel, Nahe, Rheinhessen Ministry for the Environment, Agriculture, Nutrition, Viniculture and Forestry Rhineland-Palatinate Kaiser-Friedrich-Straße 1, 55116 Mainz and Pfalz. Six of the thirteen German wine-growing regions are located in Rhineland-Palatinate [email protected] and characterize large areas of our state. www.mulewf.rlp.de Wine has been grown here since Roman times. Wine production has created unique cultural landscapes in Rhineland-Palatinate and is an important economic factor today. This is not Coordination and editors: only a result of wine production alone, which generates nearly a third of the total agricultural Dr. J. Backes*, Dr. P. Böhm, H. Gröber**, J. Jung*, Dr. E.-D. Spies*** production value of our federal state, but is also due to the growing number of tourists who come here because of the wine. -

Fulda Weser Diemel Werra Ulster Frieda Lahn Oberrhein Mittelrhein
Risikogebiet (Bezeichnung im 2. HWRM-Zyklus) Kennummer (APSFR_CD) Hydraulische Bearbeitung im: Bisherige Bezeichnung/Aufteilung im 1. Zyklus Zuständig Fulda 1. Zyklus (hessisches Pilotprojekt) mit z.T. Neubearbeitung im HWRMP-Fulda Regierungspräsidium Kassel, Standort Kassel, 2. Zyklus Abteilung III Umwelt- und Arbeitsschutz, Dezernat: 31.3 (Gewässerkennziffer 42) Oberirdische Gewässer, Hochwasserschutz (außer im Von der Mündung der Fliede in die Fulda bis zur Landesgrenze Hessen/Niedersachsen (die unteren 5 km liegen in Niedersachsen). Gewässerabschnitt innerhalb des Vogelsbergkreises). Mit Berücksichtigung der folgenden Gewässerabschnitte und Nebengewässer: Fulda (Gewässerkennziffer 42) - im Vogelsbergkreis 1. Zyklus mit Neubearbeitung im 2. Zyklus Regierungspräsidium Gießen, Dezernat: 41.2 Oberirdische Abschnitt der Fulda innerhalb des Vogelsbergkreises, von Flusskilometer 143,9 bis 168,0. Gewässer, Hochwasserschutz. Eder (Gewässerkennziffer 428) DEHE_RG_42_FUL_PE04 1. Zyklus Regierungspräsidium Kassel, Standort Kassel, Abteilung III Von der Landesgrenze zu Nordrhein-Westfalen bis zur Mündung in die Fulda. Umwelt- und Arbeitsschutz, Dezernat: 31.3 Oberirdische Gewässer, Hochwasserschutz. Haune (Gewässerkziffer 426) Von der Haunetalsperre bis zur Mündung in die Fulda. Losse (Gewässerkennziffer 4296) 1. Zyklus mit Neubearbeitung im 2. Zyklus Verlauf innerhalb des Stadtgebiets Kassel. Schwalm (Gewässerkennziffer 4288) 1. Zyklus Vom Hochwasserrückhaltebecken Heidelbach bis zur Mündung in die Eder. Weser 1. Zyklus HWRMP-Diemel und Weser -

Council CNL(14)23 Annual Progress Report on Actions Taken
Agenda Item 6.1 For Information Council CNL(14)23 Annual Progress Report on Actions Taken Under Implementation Plans for the Calendar Year 2013 EU – Germany CNL(14)23 Annual Progress Report on Actions taken under Implementation Plans for the Calendar Year 2013 The primary purposes of the Annual Progress Reports are to provide details of: • any changes to the management regime for salmon and consequent changes to the Implementation Plan; • actions that have been taken under the Implementation Plan in the previous year; • significant changes to the status of stocks, and a report on catches; and • actions taken in accordance with the provisions of the Convention These reports will be reviewed by the Council. Please complete this form and return it to the Secretariat by 1 April 2014. The annual report 2013 is structured according to the catchments of the rivers Rhine, Ems, Weser and Elbe. Party: European Union Jurisdiction/Region: Germany 1: Changes to the Implementation Plan 1.1 Describe any proposed revisions to the Implementation Plan and, where appropriate, provide a revised plan. Item 3.3 - Provide an update on progress against actions relating to Aquaculture, Introductions and Transfers and Transgenics (section 4.8 of the Implementation Plan) - has been supplemented by a new measure (A2). 1.2 Describe any major new initiatives or achievements for salmon conservation and management that you wish to highlight. Rhine ICPR The 15th Conference of Rhine Ministers held on 28th October 2013 in Basel has agreed on the following points for the rebuilding of a self-sustainable salmon population in the Rhine system in its Communiqué of Ministers (www.iksr.org / International Cooperation / Conferences of Ministers): - Salmon stocking can be reduced step by step in parts of the River Sieg system in the lower reaches of the Rhine, even though such stocking measures on the long run remain absolutely essential in the upper reaches of the Rhine, in order to increase the number of returnees and to enhance the carefully starting natural reproduction.