Food Sources Contributing to the Diet of Common and Crucian Carps in a Shallow, Temperate, Eutrophic Lake
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biogeography and Evolution of the Carassius Auratus-Complex in East
Takada et al. BMC Evolutionary Biology 2010, 10:7 http://www.biomedcentral.com/1471-2148/10/7 RESEARCH ARTICLE Open Access Biogeography and evolution of the Carassius auratus-complex in East Asia Mikumi Takada1,2*, Katsunori Tachihara1, Takeshi Kon2, Gunji Yamamoto2, Kei’ichiro Iguchi3, Masaki Miya4, Mutsumi Nishida2 Abstract Background: Carassius auratus is a primary freshwater fish with bisexual diploid and unisexual gynogenetic triploid lineages. It is distributed widely in Eurasia and is especially common in East Asia. Although several genetic studies have been conducted on C. auratus, they have not provided clear phylogenetic and evolutionary descriptions of this fish, probably due to selection bias in sampling sites and the DNA regions analysed. As the first step in clarifying the evolutionary entity of the world’s Carassius fishes, we attempted to clarify the phylogeny of C. auratus populations distributed in East Asia. Results: We conducted a detailed analysis of a large dataset of mitochondrial gene sequences [CR, 323 bp, 672 sequences (528 sequenced + 144 downloaded); CR + ND4 + ND5 + cyt b, 4669 bp in total, 53 sequences] obtained from C. auratus in East Asia. Our phylogeographic analysis revealed two superlineages, one distributed mainly among the Japanese main islands and the other in various regions in and around the Eurasian continent, including the Ryukyus and Taiwan. The two superlineages include seven lineages with high regional specificity that are composed of endemic populations indigenous to each region. The divergence time of the seven lineages was estimated to be 0.2 million years ago (Mya) by a fossil-based method and 1.0-1.9 Mya by the molecular clock method. -
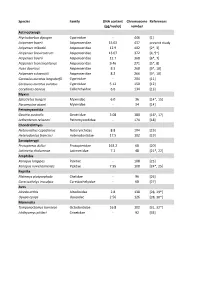
Table S2.Xlsx
Species Family DNA content Chromosome References (pg/nuclei) number Actinopterygii Ptychobarbus dipogon Cyprinidae ‐ 446 [1] Acipenser baerii Acipenseridae 15.02 437 present study Acipenser mikadoi Acipenseridae 12.9 402 [2*, 3] Acipenser brevirostrum Acipenseridae 13.07 372 [4, 5*] Acipenser baerii Acipenseridae 12.7 368 [6*, 7] Acipenser transmontanus Acipenseridae 9.46 271 [5*, 8] Huso dauricus Acipenseridae 8.3 268 [9*, 10] Acipenser schrenckii Acipenseridae 8.2 266 [9*, 10] Carassius auratus langsdorfii Cyprinidae ‐ 204 [11] Carassius auratus auratus Cyprinidae 5.12 150 [12] Corydoras aeneus Callichthyidae 6.6 134 [13] Myxini Eptatretus burgeri Myxinidae 6.0 36 [14*, 15] Paramyxine atami Myxinidae ‐ 34 [14] Petromyzontida Geotria australis Geotriidae 3.08 180 [16*, 17] Lethenteron reissneri Petromyzontidae ‐ 174 [18] Chondrichthyes Notorynchus cepedianus Notorynchidae 8.8 104 [19] Heterodontus francisci Heterodontidae 17.5 102 [19] Sarcopterygii Protopterus dolloi Protopteridae 163.2 68 [20] Latimeria chalumnae Latimeriidae 7.2 48 [21*, 22] Amphibia Xenopus longipes Pipidae ‐ 108 [23] Xenopus ruwenzoriensis Pipidae 7.95 108 [24*, 25] Reptilia Platemys platycephala Chelidae ‐ 96 [26] Carettochelys insculpta Carettochelyidae ‐ 68 [27] Aves Alcedo atthis Alcedinidae 2.8 138 [28, 29*] Upupa epops Upupidae 2.56 126 [28, 30*] Mammalia Tympanoctomys barrerae Octodontidae 16.8 102 [31, 32*] Ichthyomys pittieri Cricetidae ‐ 92 [33] References 1. Yu XY, Yu XJ. A schizothoracin fish species, Diptychus dipogon, with very high number of chromosomes Chrom Inform Serv. 1990;48:17-8. 2. Zhou H, Fujimoto T, Adachi S, Yamaha E, Arai K. Genome size variation estimated by flow cytometry in Acipenser mikadoi, Huso dauricus in relation to other species of Acipenseriformes. -

Page 1 F Fish Pathology, 46 (3), 87–90, 2011. 9 © 2011 the Japanese
魚病研究 Fish Pathology, 46 (3), 87–90, 2011. 9 © 2011 The Japanese Society of Fish Pathology Blood Fluke Infection of Cage 2 to 10 months (average of 6 months) fed with highly fat content fish, mainly chub mackerel Scomber japonicus, R eared Atlantic Bluefin Tuna A tla n tic mackerel S c o mber scombru s , European pil- Thunnus thynnus in chard C lupea pilchartus and round sardinella S a rd in e lla aurita. After this period, tunas are sacrificed in the Wes t Mediterranean floating cages a n d immediately commercialized fresh or frozen. R ocio Ruiz de Ybañez1, José Peñalver2, Among pathological problems reported in reared C arlos Martínez-Carrasco1, Laura del Río1, tuna, a blood fluke Cardicola forsteri (Digenea: 2 1 Aporocotylidae), has been pointed out as a significant Emilio María Dolores , Eduardo Berriatua risk of tuna health1). Initially identified in the Australian 1 and Pilar Muñoz * population of farmed southern BFT Thunnus maccoyii 2), this blood fluke was later reported in Atlantic BFT3–5), 1 Animal Health Department, University of Murcia, being the only one aporocotylid repo rte d s o fa r in th is Murcia 30100, Spain 2 species. Aporocotylids are parasites of marine and Livestock and Fishery Departmen t, Murcia 6) fre s h wate r fis h . Most species are located in the R egional Gov e rn men t, Murcia 30201, heart, bulbus arteriosus, ventral aorta or branchial ves- S p a in sels, although the cephalic or dorsal vessels are not uncommon habitats7). Once established, adult flukes (Recieved November 24, 2010) lay eggs whic h tra v e l to th e g ills where they lodge. -

Pest Risk Assessment for Asian Carps in Oregon
Pest Risk Assessment for Asian Carps in Oregon IDENTITY Name: Asian Carps The common usage of the term “Asian Carps” encompasses the following four species of introduced carp (there are additional species of carp native to Asia that have been introduced into the U.S. but are not commonly included under term “Asian Carps” – see text). • bighead carp (Hypophthalmichthys nobilis) • silver carp (Hypophthalmichthys molitrix) • black carp (Mylopharyngodon piceus) • grass carp (Ctenopharyngodon idella) Taxonomic Position: order Cypriniformes, family Cyprinidae [carps and minnows] The family Cyprinidae is very diverse – it includes species that feed on plankton, herbivores, omnivores, piscivores (fish eaters such as our native pike minnow) and species like the black carp whose teeth are specially modified to crush the shells of mussels and snails - and as such it can be difficult to distinguish native versus nonnative species based on a few simple characteristics. Nevertheless, the collection of nonnative species such as Asian carps should be reported to the Oregon Department of Fish and Wildlife. Positive identification is crucial and for this reason we recommend retaining the specimen if possible or documenting the catch with photographs. Well-focused images that show the whole fish from various angles as well as close-ups of the head and fins are ideal. Additional information on identification of Asian and other nonnative carps has been compiled by the US Geological Survey and can be accessed online <http://fisc.er.usgs.gov/Carp_ID/index.html>. RISK RATING SUMMARY Relative Risk Rating: HIGH Numerical Score: 6 (on a 1-9 scale) Uncertainty: Moderate This Risk Evaluation summarizes much of the information previously compiled by the USFWS in 2008. -

Biological Control of Invasive Fish and Aquatic Invertebrates: a Brief Review with Case Studies
Management of Biological Invasions (2019) Volume 10, Issue 2: 227–254 CORRECTED PROOF Review Biological control of invasive fish and aquatic invertebrates: a brief review with case studies Przemyslaw G. Bajer1,*, Ratna Ghosal1,+, Maciej Maselko2, Michael J. Smanski2, Joseph D. Lechelt1, Gretchen Hansen3 and Matthew S. Kornis4 1University of Minnesota, Minnesota Aquatic Invasive Species Research Center, Dept. of Fisheries, Wildlife, and Conservation Biology, 135 Skok Hall, 2003 Upper Buford Circle, St. Paul, MN 55108, USA 2University of Minnesota, Dept. of Biochemistry, Molecular Biology and Biophysics, 1479 Gortner Ave., Room 344, St. Paul MN 55108, USA 3Minnesota Department of Natural Resources, 500 Lafayette Road, St. Paul, MN 55155, USA 4U.S. Fish and Wildlife Service, Great Lakes Fish Tag and Recovery Laboratory, Green Bay Fish and Wildlife Conservation Office, 2661 Scott Tower Drive, New Franken, WI 54229, USA +Current Address: Biological and Life Sciences, School of Arts and Sciences, Ahmedabad University, Gujarat 380009, India Author e-mails: [email protected] (PGB), [email protected] (RG), [email protected] (MM), [email protected] (MJS), [email protected] (JDL), [email protected] (GH), [email protected] (MSK) *Corresponding author Citation: Bajer PG, Ghosal R, Maselko M, Smanski MJ, Lechelt JD, Hansen G, Abstract Kornis MS (2019) Biological control of invasive fish and aquatic invertebrates: a We review various applications of biocontrol for invasive fish and aquatic brief review with case studies. invertebrates. We adopt a broader definition of biocontrol that includes traditional Management of Biological Invasions 10(2): methods like predation and physical removal (biocontrol by humans), and modern 227–254, https://doi.org/10.3391/mbi.2019. -

Polyphyletic Origin of Ornamental Goldfish
Food and Nutrition Sciences, 2015, 6, 1005-1013 Published Online August 2015 in SciRes. http://www.scirp.org/journal/fns http://dx.doi.org/10.4236/fns.2015.611104 Polyphyletic Origin of Ornamental Goldfish Aleksandr V. Podlesnykh1, Vladimir A. Brykov1,2, Lubov A. Skurikhina1 1Far Eastern Branch of the Russian Academy of Sciences, A. V. Zhirmunsky Institute of Marine Biology, Vladivostok, Russia 2School of Natural Sciences, Far Eastern Federal University, Vladivostok, Russia Email: [email protected] Received 6 May 2015; accepted 17 August 2015; published 20 August 2015 Copyright © 2015 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Mitochondrial DNA fragment of cytb was compared in all species Carassius auratus complex and three forms of ornamental goldfish. It is shown that the phylogenetic relationships between com- plex species correspond to the existing views, based on mtDNA data and geographical distribution. All forms of ornamental goldfish have a monophyletic origin from Chinese goldfish C. auratus au- ratus. The analysis showed that three nuclear genes (rps7, GH1 and Rh) in the two forms of orna- mental goldfish (Oriental twintail goldfish and Chinese Ranchu) were almost identical C. auratus auratus genes, while all three gene in another more simple form of goldfish (common goldfish) were highly homologous to carp Cyprinus carpio nuclear genes. The obtained data suggested that in the history of aquarium goldfish breeding occurred the stage of distant hybridization between goldfish and common carp. Subsequently, the nuclear genomes of some ornamental forms could be enriched by goldfish genes (a relatively recent form as Oriental twintail goldfish and Chinese Ranchu) or common carp genes (the simplest and most ancient forms like common goldfish) as a result of multidirectional breeding and selection of aquarium goldfish various forms. -

APPENDIX M Common and Scientific Species Names
Bay du Nord Development Project Environmental Impact Statement APPENDIX M Common and Scientific Species Names Bay du Nord Development Project Environmental Impact Statement Common and Species Names Common Name Scientific Name Fish Abyssal Skate Bathyraja abyssicola Acadian Redfish Sebastes fasciatus Albacore Tuna Thunnus alalunga Alewife (or Gaspereau) Alosa pseudoharengus Alfonsino Beryx decadactylus American Eel Anguilla rostrata American Plaice Hippoglossoides platessoides American Shad Alosa sapidissima Anchovy Engraulidae (F) Arctic Char (or Charr) Salvelinus alpinus Arctic Cod Boreogadus saida Atlantic Bluefin Tuna Thunnus thynnus Atlantic Cod Gadus morhua Atlantic Halibut Hippoglossus hippoglossus Atlantic Mackerel Scomber scombrus Atlantic Salmon (landlocked: Ouananiche) Salmo salar Atlantic Saury Scomberesox saurus Atlantic Silverside Menidia menidia Atlantic Sturgeon Acipenser oxyrhynchus oxyrhynchus Atlantic Wreckfish Polyprion americanus Barndoor Skate Dipturus laevis Basking Shark Cetorhinus maximus Bigeye Tuna Thunnus obesus Black Dogfish Centroscyllium fabricii Blue Hake Antimora rostrata Blue Marlin Makaira nigricans Blue Runner Caranx crysos Blue Shark Prionace glauca Blueback Herring Alosa aestivalis Boa Dragonfish Stomias boa ferox Brook Trout Salvelinus fontinalis Brown Bullhead Catfish Ameiurus nebulosus Burbot Lota lota Capelin Mallotus villosus Cardinal Fish Apogonidae (F) Chain Pickerel Esox niger Common Grenadier Nezumia bairdii Common Lumpfish Cyclopterus lumpus Common Thresher Shark Alopias vulpinus Crucian Carp -

Electrophoretic Studies of Diploid, Triploid, and Tetraploid Forms of the Japanese Silver Crucian Carp, Carassius Auratus Langsdorfii
Japan.J.Ichthyol. 魚 類 学 雑 誌 40(1):65-75,1993 40(1):65-75,1993 Electrophoretic Studies of Diploid, Triploid, and Tetraploid Forms of the Japanese Silver Crucian Carp, Carassius auratus langsdorfii Yasushi Shimizu,1,2 Takashi Oshiro1 and Mitsuru Sakaizumi2,31 Department of Aquatic Biosciences, Tokyo University of Fisheries, 4-5-7 Konan, Minato-ku, Tokyo 108, Japan 2Department of Experimental Animal Science, The Tokyo Metropolitan Institute of Medical Science, 3-18-22 Honkomagome, Bunkyo-ku, Tokyo 113, Japan 3Present address: Department of Biology, College of General Education, Niigata University, 2-8050 Ikarashi, Niigata 950-21, Japan (Received October 29, 1992; in revisedform February 8, 1993; accepted March 22, 1993) Abstract The diploid-polyploid complex in the Japanese silver crucian carp (ginbuna), Carassius auratus langsdorfii, includes a diploid bisexual form, a triploid gynogenetic form and a tetraploid gynogenetic form. However, the origin of the polyploids remains to be clarified. Flow cytometric and electrophoretic analyses of Japanese crucian carp demonstrated that all the individuals with a two-banded pattern for an AMY-2 gene were polyploid, and 90% of the polyploids gave such a two-banded pattern. One of the two bands was not found in diploid subspecies in Japan but was present in both Korean crucian carp and goldfish. The diploid subspecies, kinbuna (C.auratus subsp.), which is distributed along the Pacific coast of eastern Japan, generated a diagnostic band of phosphoglucomutase. This band was also found in the polyploid "ginbuna" from the same region, but was observed in neither triploids nor diploids from other regions. -

A Brief Diet Analysis of Common Carp and Channel Catfish by Brent Campos
A Brief Diet Analysis of Common Carp and Channel Catfish By Brent Campos Four mainstem nonnative fish, two channel catfish (Ictalurus punctatus) and two common carp (Cyprinus carpio), were captured using hook-and-line during our research expedition down the Grand Canyon in March, 2005. The stomachs of the fish were removed and their contents analyzed. The two species of fish appeared to exhibit divergent methods of feeding, despite having very similar organisms in their stomachs. Catfish displayed a selective, possibly mid-water picking strategy, while carp appeared to be consuming benthic substrates with their prey. Figure 1. The 405 mm common carp with its abdominal cavity cut open. Note the large ovaries, which are yellow and grainy in appearance (photo: Chris Hammersmark). One common carp was caught near sunset in a small eddy near Bass Camp at river mile 108 on March 21. It was male, measured 370 mm standard length (SL) and exhibited spawning colors. Identifiable stomach contents included aquatic Coeloptra (beetles), one oligochete, many pebbles, and detritus, among other benthic inverts. The presence of numerous pebbles indicates this fish was probing substrates for food, as would be expected for this species. The second common carp was caught near the mouth of the backwater at river mile 137, which was at the separation point of the eddy that created the site’s sandbar. The carp was female, 405 mm in length (standard length), and displayed spawning colors. Her ovaries made up approximately 50% of her abdominal cavity by volume. Gut contents were comprised of 95% algae and 5% a mix of detritus and aquatic invertebrates. -

Press Release
Institute of Statistics [email protected] Rr. Vllazën Huta, www.instat.gov.al Ndërtesa 35, Hyrja 1 Tel: +355 4 2222 411 Tiranë, Kodi Postar 1017 Fax: +355 4 2222 411 Fishery Statistics 2019 Tirana, 26 June 2020: In 2019, catches in all fish categories was 15,011 tonnes from 14,875 tonnes in 2018, increasing by 0.91 %. Fig. 1 Fish catches in total 2014 – 2019 (tonnes) 16000 14,875 15,011 15000 14000 13000 12,534 12,719 12000 11,022 11,170 11000 10000 2014 2015 2016 2017 2018 2019 Catches structure by water categories Fishing water categories are: marine, brackish waters, lagoons, inland waters, aquaculture and mollusks. Two are the main categories which represent the biggest percentage of fish catches, respectively “Marine” fishing with 36.64 % and "Aquaculture" with 34.83 % followed by Inland waters with 18.46 % of the total catches. Fig. 2 Catches structure by water categories, 2019 (in percentage) 7.16 Marine Costal line 36.64 Costal lagoons 34.83 Inland waters Aquaculture Mitylus galloprovicialis 18.46 2.28 0.62 For release 26/06/2020 Continues Page 2 Fishery Statistics 2019 Annual catch changes by water category In 2019, the category “Inland waters” faced the largest increase, by 14.20 % compared to the previous year, followed by the catches of the category of “Coastal line” by 8.56 % and “Aquaculture” by 1.77 %. The aquatic category "Coastal lagoons", marked a decrease compared to a year ago, about 73.25 %, followed by the category "Mollusk" with 2.98 %. Tab.1 Catches by water category Description 2015 2016 2017 2018 2019 Fishing -

Direct Cytotoxic Activity of CD8+ T Cells Against Ichthyophthirius Multifiliis
426 Abstracts / Fish & Shellfish Immunology 91 (2019) 421e472 importance for stress effects on the immune response in teleosts. Indi- # Corresponding author. vidual aspects of the interference of stress hormones (mainly cortisol) with E-mail address: [email protected] (J. Zou). immune processes have already been reported in some bony fish. Although less studied, the catecholamines adrenaline and noradrenaline have also shown to modulate the immune response of teleost leukocytes via a and b adrenergic receptors. This study aims to expand the actual knowledge on stress-induced immune modulation, in order to evaluate O-014. the effects of stress on the immune system of maraena whitefish (Cor- Direct cytotoxic activity of CD8þ T cells against Ichthyophthirius egonus maraena). This salmonid fish is highly sensitive to stress compared multifiliis in ginbuna crucian carp, Carassius auratus langsdorfii to other salmonid species long adapted to aquaculture. To this end, a large set of specific primers was designed for reverse-transcription quantitative Masaki Sukeda, Koumei Shiota, Takahiro Nagasawa, Miki Nakao, real-time PCR (RT-qPCR) analyses. The primer panel included cell-specific Tomonori Somamoto#. marker genes characterizing the distinct cell populations in the head kidney of C. maraena, which had been sorted using flow cytometry. In Laboratory of Marine Biochemistry, Department of Bioscience and addition, we analysed the expression of catecholamine and cortisol re- Biotechnology, Graduate School of Bioresource and Bioenvironmental ceptors in each population, in order to define the repertoire of stress- Sciences, Kyushu University, Fukuoka, Japan related modulators present in the cells. In the next step, we performed a series of in vitro stimulations of head kidney leukocytes to study the Abstract expression of genes involved in immune activation and acute phase A line of studies has shown that several humoral immune factors including together with catecholamine and cortisol receptors. -

In Vitro Characteristics of Cyprinid Herpesvirus 2: Effect of Kidney Extract Supplementation on Growth
Vol. 115: 223–232, 2015 DISEASES OF AQUATIC ORGANISMS Published August 20 doi: 10.3354/dao02885 Dis Aquat Org In vitro characteristics of cyprinid herpesvirus 2: effect of kidney extract supplementation on growth Tomoya Shibata1, Azusa Nanjo1, Masato Saito1, Keisuke Yoshii1, Takafumi Ito2, Teruyuki Nakanishi3, Takashi Sakamoto1, Motohiko Sano1,* 1Faculty of Marine Science, Tokyo University of Marine Science and Technology, Minato, Tokyo 108-8477, Japan 2Aquatic Animal Health Division, National Research Institute of Aquaculture, Fisheries Research Agency, Tamaki, Mie 519-0423, Japan 3Department of Veterinary Medicine, Nihon University, Fujisawa, Kanagawa 252-0880, Japan ABSTRACT: Herpesviral hematopoietic necrosis caused by goldfish hematopoietic necrosis virus (now identified as cyprinid herpesvirus 2, CyHV-2) has contributed to economic losses in goldfish Carassius auratus culture and is becoming a major obstacle in Prussian carp C. gibelio aquacul- ture in China. Several reports have described difficulties in culturing the virus, with the total loss of infectivity within several passages in cell culture. We succeeded in propagating CyHV-2 with a high infectious titer in a RyuF-2 cell line newly derived from the fin of the Ryukin goldfish variety using culture medium supplemented with 0.2% healthy goldfish kidney extract. The addition of kidney extract to the medium enabled rapid virus growth, resulting in the completion of cyto- pathic effect (CPE) within 4 to 6 d at 25°C. The extract also enabled reproducible virus culture 5−6 −1 with a titer of 10 TCID50 ml . The virus cultured using this protocol showed pathogenicity in goldfish after intraperitoneal injection. The virus grew in RyuF-2 cells at 15, 20, 25, 30, and 32°C but not at 34°C or higher.