19021105 RS Medex ENG.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Praying Mantises of the Maltese Islands: Distribution and Ecology (Mantodea)
Fragmenta entomologica, 52 (2): 341–348 (2020) eISSN: 2284-4880 (online version) pISSN: 0429-288X (print version) Research article Submitted: September 5th, 2020 - Accepted: September 28th, 2020 - Published: November 15th, 2020 The praying mantises of the Maltese Islands: distribution and ecology (Mantodea) Thomas CASSAR Department of Biology, Faculty of Science, University of Malta - Msida MSD 2080, Malta - [email protected] Abstract This study presents a species account of the mantises of the Maltese Islands, including notes on the ecology and distribution of each spe- cies. A total of three species are known to exist locally; Ameles spallanzania (Rossi, 1792), Mantis religiosa (Linnaeus, 1758) and Riv- etina baetica Rambur, 1839. The presence of Ameles decolor (Charpentier, 1825) cannot be confirmed by any recently collected mate- rial, but the species is not excluded from the Maltese entomofauna. Two doubtful records are also discussed. All species present in the archipelago are typically found in Southern Europe and the Mediterranean basin. Key words: mantids, Malta, Mediterranean. Introduction “Devil’s mare” respectively. Though Gulia (1858) men- tions Iris oratoria and Blepharopsis mendica, much doubt The Maltese archipelago is composed of a number of can be cast on these identifications. Maltese mantises were small, low islands situated in the centre of the Mediter- not mentioned again in literature until the work of Valletta ranean Sea, aligned in a North-West to South-East direc- (1954), at that time including two species - Mantis religi- tion. The total area of the archipelago amounts to 314 km2, osa and Ameles spallanzania, along with a list of Orthop- and they lie approximately 96 km to the south of Sicily tera. -

Successful Fight Wound Management in a Tunisian Eyed Lizard (Timon Pater)
EXOTICS Case study: successful fight wound management in a Tunisian eyed lizard (Timon pater) Extensive fight wounds are common in co-habiting reptiles. This case study describes the management of a large fight wound in a 1-year-old entire female Tunisian eyed lizard. Primary closure was initially attempted but subsequent postoperative infection and wound breakdown led to successful management by secondary intention healing. This case demonstrates the amazing capacity for healing of large integument defects in lizards that receive appropriate medical support. https://doi.org/10.12968/coan.2019.0044 Krissy Green BVM&S BSc (Hons) MSc CertAVP (ZooMed) MRCVS, RCVS Recognised Advanced Practitioner, Ark Veterinary Clinics, 479-481 Main Street, Coatbridge, ML5 3RD. [email protected] Key words: fight wound management | primary closure | reptile | secondary wound healing unisian eyed lizards (Timon pater), a member of the stratum corneum layers under the old skin. Enzymes dissolve the lacertid family, are native to North Africa. Inquisitive, connection between the new and old intermediate zones and the relatively easy to care for and tame, they make space fills with lymph, resulting in shedding of old skin. During rewarding pets. Groups of males and male-female times of growth and regeneration the resting phase time decreases Tpairs cohabit successively; however, housing females together and reptiles shed more frequently. often results in physical aggression and fight wounds. This case study looks at the management of an extensive full skin thickness Reptile wound healing fight wound covering two-thirds of the dorsolateral body wall of a Although the stages of mammalian and reptile wound healing 1-year-old entire female Tunisian eyed lizard. -

Timon Lepidus (Daudin, 1802) Timon Lepidus (Daudin, 1802) Lézard Ocellé
Timon lepidus (Daudin, 1802) Timon lepidus (Daudin, 1802) Lézard ocellé Ecologie et statut de l'espèce Section révisée et complétée par Philippe Geniez. cd_ref 79273 Famille Lacertidae Aire de répartition mondiale : Le Lézard ocellé (Timon lepidus, anciennement connu sous le nom de Lacerta lepida fait partie d'un petit genre de la famille des Lacertidae comprenant six espèces, 4 distribuées dans l'ouest du bassin méditerranéen (le groupe de Timon lepidus) et deux dans l'est (groupe de Timon princeps). Sur la base de caractéristiques morphologiques (taille, coloration, forme des dents, etc.), trois sous-espèces sont actuellement retenues au sein de l'espèce lepidus bien que plusieurs auteurs ne les reconnaissent pas toutes : -Timon l. lepidus (Daudin 1802), occupe la majeure partie de la péninsule Ibérique, la moitié sud et l'ouest de la France jusqu'à l'extrême nord-ouest de l'Italie (Cheylan & Grillet 2004, 2005 ; Salvidio et al, 2004). -Timon l. ibericus López-Seoane, 1884 est localisé en Galice (nord-ouest de l’Espagne) et dans le nord du Portugal. -Timon l. oteroi Castroviejo & Matéo, 1998 concerne une population insulaire localisée sur l'île de Sálvora en Galice. Des études phylogénétiques récentes révèlent cependant l'existence de cinq lignées génétiquement et géographiquement bien distinctes au sein de la péninsule Ibérique dont l’une d’elle, la « lignée », la plus répandue, est celle qui est présente en France et en Italie (Miraldo et al., 2011). La sous-espèce oteroi apparaît comme une population insulaire présentant quelques caractéristiques morphologiques liée à l’insularité mais qui entre clairement dans la « lignée » (T. -

Khalladi-Bpp Anexes-Arabic.Pdf
Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 107 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 108 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 109 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 110 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 111 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 112 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 113 The IUCN Red List Categories and Criteria are intended to be an easily and widely understood system for classifying species at high risk of global extinction. The IUCN Red List is categorized in the following Categories: • Extinct (EX): A taxon is Extinct when there is no reasonable doubt that the last individual has died. A taxon is presumed Extinct when exhaustive surveys in known and/or expected habitat, at appropriate times (diurnal, seasonal, annual), throughout its historic range have failed to record an individual. Surveys should be over a time frame appropriate to the taxon’s life cycle and life form. Khalladi Windfarm and Power Line Projects 114 Biodiversity Protection Plan, July 2015 • Extinct in the Wild (EW): A taxon is Extinct in the Wild when it is known only to survive in cultivation, in captivity or as a naturalized population (or populations) well outside the past range. A taxon is presumed Extinct in the Wild when exhaustive surveys in known and/or expected habitat, at appropriate times (diurnal, seasonal, annual), throughout its historic range have failed to record an individual. -
B I R D W a T C H I N G •
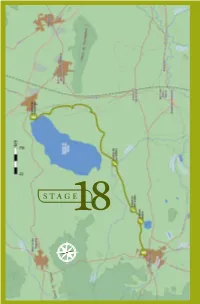
18 . F UENTE DE PIEDRA - CAMPILLOS STAGE18 E S N O 158 BIRDWATCHING • GR-249 Great Path of Malaga F UENTE DE PIEDRA - CAMPILLOS 18 . STAGE 18 Fuente de Piedra - Campillos L O C A T I O N he José Antonio Valverde Visitor´s Centre at the TReserva Natural de la Laguna de Fuente de Piedra is the starting point of Stage 18. Taking the direction south around the taken up mainly by olive trees and grain. eastern side of the salt water lagoon This type of environment will continue to you will be walking through farmland the end of this stage and it determines until the end of this stage in Campillos the species of birds which can be seen village. The 15, 7 km of Stage 18 will here. You will be crossing a stream and allow you to discover this wetland well then walking along the two lakes which known at national level in Spain, and will make your Stage 18 bird list fill cultivated farmland which creates a up with highly desirable species. The steppe-like environment. combination of wetland and steppe creates very valuable habitats with DESCRIPTION a rare composition of taxa unique at European level. ABOUT THE BIRDLIFE: Stage 18 begins at the northern tip HIGHLIGHTED SPECIES of the lagoon where you take direction Neither the length, diffi culty level or south through agricultural environment, elevation gain of this stage is particularly DID YOU KNOW? ecilio Garcia de la Leña, in Conversation 9th of “Historical Conversations of Malaga” published by Cristóbal Medina Conde (1726-1798) and entitled «About Animal Kingdom of Malaga and some Places in its Bishopric», Ccomments: «…In some lagoons, along sea shores and river banks some large and beautiful birds breed, called Flamingos and Phoenicopteros according to the ancients...», after a description of the bird´s anatomy he adds: «The Romans appreciated the bird greatly, especially its tongue which was served as an exquisite dish…». -

(Mantodea) from Pelješac Peninsula, Southern Croatia
Entomol. Croat. 2014, Vol. 18. Num 1–2: 7–11 MANTIDS (MANTODEA) FROM PELJEŠAC PENINSULA, SOUTHERN CROATIA Jerzy Romanowski and Mateusz Romanowski Faculty of Biology and Environmental Sciences, Cardinal Stefan Wyszyński University, Wóycickiego 1/3, 01-938, Warsaw, Poland [email protected] Surveys for mantids were undertaken at 9 sites, located along the northern and southern coasts and in the interior of Pelješac Peninsula, southern Croatia. Sweep nettings and visual observations were carried out from 27 July 2013 till 8 August 2013. Five mantid species from four genera were observed, including Ameles cf. decolor (Charpentier, 1825), A. spallanzania (Rossi, 1792), Iris oratoria (Linnaeus, 1758), Mantis religiosa Linnaeus, 1758, and Empusa fasciata Brullé, 1832. The first exact records of the Mediterranean Mantis, I. oratoria in conti- nental Croatia are reported. The need for a detailed study on the sympatric distribution of A. decolor and A. heldreichi in Croatia is addressed. Key words: Mantodea, Iris oratoria, faunistics, new records, Croatia, Bal- kan Peninsula J. ROMANOWSKI i M. ROMANOWSKI: Bogomoljke (MANTODEA) na poluotoku Pelješcu (južna Hrvatska). Entomol. Croat. Vol. 18. Num. 1–2: 7–11 Istraživanja bogomoljki provedena su na 9 lokaliteta smještenih duž sje- verne i južne obale te unutrašnjosti poluotoka Pelješca (južna Hrvatska). Teh- nikom košnje pomoću entomološke mrežice i vizualnim opažanjima bogomolj- ke su proučavane od 27. srpnja do 8. kolovoza 2013. godine. Zabilježeno je 5 vrsta koje pripadaju u 4 roda, uključujući vrste Ameles cf. decolor (Charpentier, 1825), A. spallanzania (Rossi, 1792), Iris oratoria (Linnaeus, 1758), Mantis religiosa Linnaeus, 1758 I Empusa fasciata Brullé, 1832. U radu se navodi i prva točna evidencija mediteranske vrste bogomoljke I. -
![Catálogo Electrónico De Los Cicindelidae Y Carabidae De La Península Ibérica (Coleoptera, Caraboidea) [Versión 12•2020]](https://docslib.b-cdn.net/cover/5571/cat%C3%A1logo-electr%C3%B3nico-de-los-cicindelidae-y-carabidae-de-la-pen%C3%ADnsula-ib%C3%A9rica-coleoptera-caraboidea-versi%C3%B3n-12-2020-345571.webp)
Catálogo Electrónico De Los Cicindelidae Y Carabidae De La Península Ibérica (Coleoptera, Caraboidea) [Versión 12•2020]
Monografías electrónicas SEA, vol. 9 (2020) ▪ Sociedad Entomológica Aragonesa (S.E.A.) 1 Catálogo electrónico de los Cicindelidae y Carabidae de la Península Ibérica (Coleoptera, Caraboidea) [Versión 12•2020] José Serrano Tercera parte: Bibliografía Para facilitar el acceso a la información y la localización de obras, la presente sección se divide en dos bloques. En el primero se reproduce el listado bibliográfico recogido hasta 2013 en el anterior Catálogo impreso del autor. Se incluye la numeración original. En el segundo bloque (página 35 de esta sección) se incluye las obras posteriores y se subsanan algunas ausencias anteriorres a 2013. 1. Bibliografía incluida en SERRANO J. (2013) New catalogue of the family Carabidae of the Iberian Peninsula (Coleoptera). Ediciones de la Universidad de Murcia, 192 pp. Obras de conjunto sobre la taxonomía de los Carabidae de la Península Ibérica, Francia y Marruecos / General works on the taxonomy of the family Carabidae from the Iberian Pen‐ insula, France and Morocco 1. ANTOINE M. 1955-1962. Coléoptères Carabiques du Maroc. Mem. Soc. Sci. Nat. Phys. Maroc (N.S. Zoologie) Rabat 1 (1955, 1er partie): 1-177; 3 (1957, 2ème partie): 178-314; 6 (1959, 3ème partie): 315-464; 8 (1961, 4ème partie): 467-537; 9 (1962, 5ème partie): 538-692. 2. DE LA FUENTE J.M. 1927. Tablas analíticas para la clasificación de los coleópteros de la Península Ibérica. Adephaga: 1 Cicindelidae, 11 Carabidae. 1. Bosch, Barcelona, 415 pp. 3. JEANNEL R. 1941-1949. Coléoptères Carabiques. Faune de France, 39 (1941): 1-571; 40 (1942): 572-1173; 51 (1949, Supplément): 1- 51. Lechevalier, París. -

Amphibiaweb's Illustrated Amphibians of the Earth
AmphibiaWeb's Illustrated Amphibians of the Earth Created and Illustrated by the 2020-2021 AmphibiaWeb URAP Team: Alice Drozd, Arjun Mehta, Ash Reining, Kira Wiesinger, and Ann T. Chang This introduction to amphibians was written by University of California, Berkeley AmphibiaWeb Undergraduate Research Apprentices for people who love amphibians. Thank you to the many AmphibiaWeb apprentices over the last 21 years for their efforts. Edited by members of the AmphibiaWeb Steering Committee CC BY-NC-SA 2 Dedicated in loving memory of David B. Wake Founding Director of AmphibiaWeb (8 June 1936 - 29 April 2021) Dave Wake was a dedicated amphibian biologist who mentored and educated countless people. With the launch of AmphibiaWeb in 2000, Dave sought to bring the conservation science and basic fact-based biology of all amphibians to a single place where everyone could access the information freely. Until his last day, David remained a tirelessly dedicated scientist and ally of the amphibians of the world. 3 Table of Contents What are Amphibians? Their Characteristics ...................................................................................... 7 Orders of Amphibians.................................................................................... 7 Where are Amphibians? Where are Amphibians? ............................................................................... 9 What are Bioregions? ..................................................................................10 Conservation of Amphibians Why Save Amphibians? ............................................................................. -

Molekularno-Filogenetičke I Morfološke Značajke Zelenih Žaba (Rod Pelophylax) U Hrvatskoj
Molekularno-filogenetičke i morfološke značajke zelenih žaba (rod Pelophylax) u Hrvatskoj Schmidt, Bruno Master's thesis / Diplomski rad 2018 Degree Grantor / Ustanova koja je dodijelila akademski / stručni stupanj: University of Zagreb, Faculty of Science / Sveučilište u Zagrebu, Prirodoslovno-matematički fakultet Permanent link / Trajna poveznica: https://urn.nsk.hr/urn:nbn:hr:217:811997 Rights / Prava: In copyright Download date / Datum preuzimanja: 2021-10-04 Repository / Repozitorij: Repository of Faculty of Science - University of Zagreb Sveučilište u Zagrebu Prirodoslovno – matematički fakultet Biološki odsjek Bruno Schmidt Molekularno–filogenetičke i morfološke značajke zelenih žaba (rod Pelophylax) u Hrvatskoj Diplomski rad Zagreb, 2018. Ovaj rad izrađen je na Zoologijskom zavodu Prirodoslovno-matematičkog fakulteta Sveučilišta u Zagrebu, pod vodstvom prof. dr. sc. Görana Klobučara i pomoćnim voditeljstvom dr. sc. Mišela Jelića. Rad je predan na ocjenu Biološkom odsjeku Prirodoslovnog-matematičkog fakulteta Sveučilišta u Zagrebu radi stjecanja zvanja magistra znanosti o okolišu. 2 Najljepše zahvaljujem mentoru dr. sc. Göranu Klobučaru na ukazanom povjerenju, stručnom vodstvu te vremenu i trudu kojeg je uložio. Najveća hvala dr. sc. Mišelu Jeliću koji me vodio kroz ovo istraživanje, rješavao sve probleme i prepreke, značajno unaprijedio moje znanje i pokazao mi kako se pravilno izvodi znanstveno istraživanje. Velika hvala svim starim i novim prijateljima koje stekao tijekom svog akademskog obrazovanja, koji su mi pružili potporu -

Tail Breakage and Predatory Pressure Upon Two Invasive Snakes (Serpentes: Colubridae) at Two Islands in the Western Mediterranean
Canadian Journal of Zoology Tail breakage and predatory pressure upon two invasive snakes (Serpentes: Colubridae) at two islands in the Western Mediterranean Journal: Canadian Journal of Zoology Manuscript ID cjz-2020-0261.R2 Manuscript Type: Article Date Submitted by the 17-Jan-2021 Author: Complete List of Authors: Febrer-Serra, Maria; University of the Balearic Islands Lassnig, Nil; University of the Balearic Islands Colomar, Victor; Consorci per a la Recuperació de la Fauna de les Illes Balears Draft Sureda Gomila, Antoni; University of the Balearic Islands; Carlos III Health Institute, CIBEROBC Pinya Fernández, Samuel; University of the Balearic Islands, Biology Is your manuscript invited for consideration in a Special Not applicable (regular submission) Issue?: Zamenis scalaris, Hemorrhois hippocrepis, invasive snakes, predatory Keyword: pressure, Balearic Islands, frequency of tail breakage © The Author(s) or their Institution(s) Page 1 of 34 Canadian Journal of Zoology 1 Tail breakage and predatory pressure upon two invasive snakes (Serpentes: 2 Colubridae) at two islands in the Western Mediterranean 3 4 M. Febrer-Serra, N. Lassnig, V. Colomar, A. Sureda, S. Pinya* 5 6 M. Febrer-Serra. Interdisciplinary Ecology Group. University of the Balearic Islands, 7 Ctra. Valldemossa km 7.5, 07122 Palma, Balearic Islands, Spain. E-mail address: 8 [email protected]. 9 N. Lassnig. Interdisciplinary Ecology Group. University of the Balearic Islands, Ctra. 10 Valldemossa km 7.5, 07122 Palma, Balearic Islands, Spain. E-mail address: 11 [email protected]. 12 V. Colomar. Consortium for the RecoveryDraft of Fauna of the Balearic Islands (COFIB). 13 Government of the Balearic Islands, Spain. -

(Dictyoptera: Mantodea) Fauna of Aspat (Strobilos), Bodrum, Mugla, Western Turkey
Research Article Bartın University International Journal of Natural and Applied Sciences JONAS, 3(2): 103-107 e-ISSN: 2667-5048 31 Aralık/December, 2020 A CONTRIBUTION TO THE KNOWLEDGE OF THE EMPUSIDAE, TARACHODIDAE AND MANTIDAE (DICTYOPTERA: MANTODEA) FAUNA OF ASPAT (STROBILOS), BODRUM, MUGLA, WESTERN TURKEY Nilay Gülperçin1*, Abbas Mol2, Serdar Tezcan3 1Natural History Application and Research Center, Ege University, Bornova, Izmir, Turkey 2 Health Academy, Deparment of Emergency Aid and Disaster Management, Aksaray University, Aksaray, Turkey 3Department of Plant Protection, Faculty of Agriculture, Ege University, Bornova Izmir, Turkey Abstract This paper maintains data about the Mantodea (Dictyoptera) fauna from Aspat (Strobilos) province of Bodrum, Muğla, Western Turkey. Species were collected using different methods namely, handpicking on vegetation, handpicking on the ground, handpicking under stone, light trap, bait trap and sweep net sampling. Sampling took place at two weeks’ intervals during the years of 2008 and 2009. At the end of this research, three species belonging to three families of Mantodea were specified. Those are Empusa fasciata Brullé, 1832 (Empusidae), Iris oratoria (Linnaeus, 1758) (Tarachodidae) and Mantis religiosa (Linnaeus, 1758) (Mantidae). Sweeping net is the effective method (40.48%)in sampling and light trap (35.71%) method followed it. All three species were sampled in both years. E. fasciata was sampled in March-May, while I. oratoria was sampled in March-December and M, religiosa was sampled in June-November. Among those species Iris oratoria was the most abundant one. All these species have been recorded for the first time from Muğla province of Turkey. Keywords: Empusidae, Tarachodidae, Mantidae, Mantodea, Dictyoptera, fauna, Turkey 1. -

July 2020 Riverside Nature Notes
July 2020 Riverside Nature Notes Dear Members and Friends... by Becky Etzler, Executive Director If you stopped by in the past We are fortunate to have such a wonderful week or so, you will have noticed family of supporters. I have to give a shout out that the Riverside Nature Center to the staff, Riverside Guides, meadow tenders, is fully open and welcoming volunteers, Kerrville Chapter of the Native Plant visitors. There were no banners, Society, Hill Country Master Naturalists, the fireworks, bullhorns or grand Board of Directors and our RNC Members. Each opening celebrations announcing of you have made this difficult time much more our reopening. Let’s call it a “soft bearable, even if we haven’t been able to hug. opening”. Let’s all keep a positive attitude and follow the The staff and I wanted to quietly put to test our example of a wonderfully wise woman, Maggie plans and protocols. Can we control the number Tatum: of people inside? Is our cleaning and sanitizing methods sufficient? Are visitors amenable to our recommendations of mask wearing and physical FRIENDS by Maggie Tatum distancing? Are we aware of all the possible touch points and have we removed potentially Two green plastic chairs hazardous or hard to clean displays? Do we have Underneath the trees, adequate staff and volunteer coverage to keep Seen from my breakfast window. up with cleaning protocols and still provide an They are at ease, engaging experience for our visitors? Framed by soft grey fence. A tranquil composition. Many hours were spent discussing and formulating solutions to all of these questions.