Highlights of Prescribing Information
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

September 11, 2018 DUR Minutes
Maine Department of Health and Human Services PAUL R. LEPAGE MaineCare Services BETHANY L. HAMM GOVERNOR Pharmacy Unit ACTING COMMISSIONER 11 State House Station Augusta, Maine 04333-0011 TO: Maine Drug Utilization Review Board DATE: 9/14/2018 RE: Maine DUR Board Meeting minutes from September 11, 2018 ATTENDANCE PRESENT ABSENT EXCUSED Linda Glass, MD X Lisa Wendler, Pharm. D., Clinical Pharmacy Specialist, X Maine Medical CTR Mike Antoniello, MD X Kathleen Polonchek, MD X Kenneth McCall, PharmD X Steve Diaz, MD X Erin Ackley, PharmD. X Corinn Martineau, PharmD. X Non –Voting Mike Ouellette, R.Ph., Change Healthcare X Jeffery Barkin, MD, Change Healthcare X Christopher Pezzullo, State Health Officer DHHS, DO X Jill Kingsbury, MaineCare Pharmacy Director X Guests of the Board: Ed Bosshart, PharmD, Jeff Caulfield, Lead Epidemiologist for Viral Infections from CDC: Discussed HCV treatment. CALL TO ORDER: 5:30PM Jill Kingsbury called the meeting to order at 5:30 PM. PUBLIC COMMENTS Robert Mead from Pfizer: Highlighted the attributes of Retacrit. Jane Guo from Otsuka: Highlighted the attributes of Jynarque. OLD BUSINESS DUR MINUTES The June DUR meeting minutes were accepted as written. MAINECARE UPDATE No update at this time. NEW BUSINESS INTRODUCTION: USE OF CHRONIC TRIPTANS The use of triptans has become standard of care for the treatment of acute migraine headaches, given their effectiveness, safety and tolerability. However, like many medications used to treat migraine, overuse renders them less effective. Additionally, rebound headaches from triptan overuse is common. For patients who experience frequent headaches, or whose headaches are long lasting or chronic, use of headache prophylactic medications are recommended by several medical associations, including the American Headache Society and the American Academy of Neurology. -

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

TEXAS MEDICAID Clinical Edit Prior Authorization Epoetin Alfa (PROCRIT)
TEXAS MEDICAID Clinical Edit Prior Authorization epoetin alfa (PROCRIT) STEP 1: CLEARLY PRINT AND COMPLETE TO EXPEDITE PROCESSING Date: Prescriber First & Last Name: Patient First & Last Name: Prescriber NPI: Patient Address: Prescriber Address: Patient ID: Prescriber Phone: Patient Date of Birth: Prescriber Fax: STEP 2: MEDICATION INFORMATION Medication Requested (Name): Quantity Requested: Dose Requested: Dosing Instructions: Patient’s Primary Diagnosis: ____________________________________ ICD 10 Code: __________ Please indicate ONE (1) of the following: OR STAR / STAR KIDS client (Go to Step 3 - PDL PA Criteria Applies) OR CHIP / PERINATE client (Go to Step 4) STEP 3: PDL PRIOR AUTHORIZATION CRITERIA FOR NON-PREFERRED PRODUCT 1. Has the client failed a 30-day treatment trial with at least 1 preferred agent in the last 180 days? Yes (Go to Step 4 Question 1) No (Go to #2) 2. Is there a documented allergy or contraindication to preferred agents in this class? Yes (Go to Step 4 Question 1) No (Go to #3) 3. Is the drug necessary for treatment of stage-4 advanced metastatic cancer and associated conditions? Yes (Go to Step 4 Question 1) No (Deny) Rev. 11/18/2020 Page 1 of 3 Version 1.5 STEP 4: CLINICAL PRIOR AUTHORIZATION CRITERIA 1. Does the client have a diagnosis of chronic renal failure in the last 730 days? Yes (Go to #7) No (Go to #2) 2. Does the client have a diagnosis of cancer in the last 730 days? Yes (Go to #3) No (Go to #5) 3. Does the client have a history of an antineoplastic agent in the last 30 days? Examples of antineoplastic -

Myelodysplastic Syndromes
MYELODYSPLASTIC SYNDROMES TREATMENT REGIMENS (Part 1 of 3) Clinical Trials: The National Comprehensive Cancer Network recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection, dosing, administration, and the management of related adverse events can be a complex process that should be handled by an experienced healthcare team. Clinicians must choose and verify treatment options based on the individual patient; drug dose modifications and supportive care interventions should be administered accordingly. The cancer treatment regimens below may include both U.S. Food and Drug Administration-approved and unapproved indications/regimens. These regimens are only provided to supplement the latest treatment strategies. These Guidelines are a work in progress that may be refined as often as new significant data becomes available. The NCCN Guidelines® are a consensus statement of its authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult any NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way. First-Line Treatment1 Note: All recommendations are Category 2A unless otherwise indicated. Relatively Lower-Risk Patientsa,b Symptomatic Anemia With del(5q) ± One Other Cytogenetic Abnormality (except those involving chromosome 7) REGIMEN DOSING Lenalidomide2-5,c Days 1–21: Lenalidomide 10mg orally once daily. Repeat cycle every 28 days (or 28 days monthly). Assess response 2–4 months after initiation of treatment. -

UWHC Guidelines for the Use of Darbepoetin and Epoetin
Use of Darbepoetin and Epoetin in Non-Nephrology Patients – Adult/Pediatric – Inpatient/Ambulatory Clinical Practice Guideline Table of Contents Executive Summary ......................................................................................... 3 Scope ............................................................................................................. 7 Methodology ................................................................................................... 7 Definitions (optional): ...................................................................................... 8 Introduction.................................................................................................... 8 Recommendations........................................................................................... 9 UW Health Implementation............................................................................ 19 References ................................................................................................... 19 Note: Active Table of Contents Click to follow link CPG Contact for Changes: Name: Philip Trapskin, PharmD, BCPS, Manager, DPP Phone Number: 608-263-1328 Email address: [email protected] CPG Contact for Content: Name: Jason Bergsbaken, PharmD Phone Number: 608-265-0341 Email address: [email protected] Copyright © 2015 University of Wisconsin Hospitals and Clinics Authority Contact: [email protected] Vermeulen, [email protected] Last Revised: 07/2015 Guideline Authors: Jason Bergsbaken, PharmD Coordinating -

Dr. Winegarden Presentation
Empowering Market Competition through Biosimilars Presentation to National Council of Insurance Legislators (NCOIL) 2019 Summer Meeting Newport Beach, California July 12, 2019 Total Savings (in millions) 25% 50% 75% Originator Total Annual Savings for Current, 25%, Drug Class Current Biosimilar Biosimilar Biosimilar Biologic 50%, and 75% Biosimilar Share Share Share Share Scenarios Compared to All-Originator Infliximab Remicade $79.4 $318.2 $636.5 $954.7 Biologic Baseline Pegfilgrastim Neulasta $21.8 $121.9 $243.8 $365.7 Filgrastim Neupogen $152.1 $152.1 $152.1 $206.8 Epoetin Alfa Epogen & Procrit $0.5 $8.4 $16.9 $25.3 Bevacizumab Avastin $0.0 $199.2 $398.5 $597.7 Trastuzumab Herceptin $0.0 $208.0 $415.9 $623.9 • Biosimilars price discounts expected 30% - Rituxumab Rituxan $0.0 $280.6 $561.2 $841.8 40% off originator biologic Etanercept Enbrel $0.0 $324.0 $648.0 $972.1 • Forthcoming study estimates possible Adalimunab Humira $0.0 $861.1 $1,722.1 $2,583.2 health care savings from wider adoption of biosimilars. GRAND TOTAL $253.8 $2,473.6 $4,795.0 $7,171.2 • Estimates are based on the average sales price (ASP) data that are effective from April 2019 through June 2019 and rolling 12-month volume data through February 2019. Biosimilars have no “clinically meaningful difference” in safety, purity, • Methodology: compare potential savings to hypothetical all-originator biologic scenario and effectiveness relative to its reference originator biologic. Just like • Over 10 years, potential savings of $24.7 generic medicines, the benefits of biosimilars are the substantial price billion, $48.0 billion, and $71.7 billion respectively. -
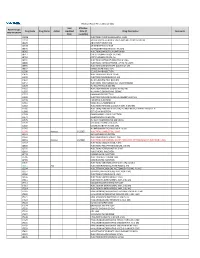
2021 Prior Authorization List Part B Appendix a (PDF)
Medicare Part B PA List Effective 2021 Last Effective Part B Drugs: Drug Code Drug Name Action Updated Date (if Drug Description Comments STEP THERAPY Date available) C9050 INJECTION, EMAPALUMAB-LZSG, 1 MG C9122 MOMETASONE FUROATE SINUS IMPLANT 10 MCG SINUVA J0129 ABATACEPT INJECTION J0178 AFLIBERCEPT INJECTION J0570 BUPRENORPHINE IMPLANT 74.2MG J0585 INJECTION,ONABOTULINUMTOXINA J0717 CERTOLIZUMAB PEGOL INJ 1MG J0718 CERTOLIZUMAB PEGOL INJ J0791 INJECTION CRIZANLIZUMAB-TMCA 5 MG J0800 INJECTION, CORTICOTROPIN, UP TO 40 UNITS J0896 INJECTION LUSPATERCEPT-AAMT 0.25 MG J0897 DENOSUMAB INJECTION J1300 ECULIZUMAB INJECTION J1428 INJECTION ETEPLIRSEN 10 MG J1429 INJECTION GOLODIRSEN 10 MG J1442 INJ FILGRASTIM EXCL BIOSIMIL J1447 INJECTION, TBO-FILGRASTIM, 1 MICROGRAM J1459 INJ IVIG PRIVIGEN 500 MG J1555 INJECTION IMMUNE GLOBULIN 100 MG J1556 INJ, IMM GLOB BIVIGAM, 500MG J1557 GAMMAPLEX INJECTION J1558 INJECTION IMMUNE GLOBULIN XEMBIFY 100 MG J1559 HIZENTRA INJECTION J1561 GAMUNEX-C/GAMMAKED J1562 INJECTION; IMMUNE GLOBULIN 10%, 5 GRAMS J1566 INJECTION, IMMUNE GLOBULIN, INTRAVENOUS, LYOPHILIZED (E.G. P J1568 OCTAGAM INJECTION J1569 GAMMAGARD LIQUID INJECTION J1572 FLEBOGAMMA INJECTION J1575 INJ IG/HYALURONIDASE 100 MG IG J1599 IVIG NON-LYOPHILIZED, NOS J1602 GOLIMUMAB FOR IV USE 1MG J1745 INJ INFLIXIMAB EXCL BIOSIMILR 10 MG J1930 Remove 1/1/2021 INJECTION, LANREOTIDE, 1 MG J2323 NATALIZUMAB INJECTION J2350 INJECTION OCRELIZUMAB 1 MG J2353 Remove 1/1/2021 INJECTION, OCTREOTIDE, DEPOT FORM FOR INTRAMUSCULAR INJECTION, 1 MG J2357 INJECTION, OMALIZUMAB, -

OMONTYS® Safely and Effectively
HIGHLIGHTS OF PRESCRIBING INFORMATION ---------------------DOSAGE FORMS AND STRENGTHS---------------------- These highlights do not include all the information needed to use Dosage Form Strengths OMONTYS® safely and effectively. See full prescribing information for OMONTYS. Single use vials 2 mg/0.5 mL, 3 mg/0.5 mL, (preservative-free) 4 mg/0.5 mL, 5 mg/0.5 mL, and OMONTYS® (peginesatide) Injection, 6 mg/0.5 mL for intravenous or subcutaneous use Single use pre-filled syringes 1 mg/0.5 mL, 2 mg/0.5 mL, Initial U.S. Approval: 2012 (preservative-free) 3 mg/0.5 mL, 4 mg/0.5 mL, 5 mg/0.5 mL, and 6 mg/0.5 mL WARNING: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL Multiple use vials 10 mg/mL and 20 mg/2 mL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, (with preservative) THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE -------------------------------CONTRAINDICATIONS------------------------------ See full prescribing information for complete boxed warning. Uncontrolled hypertension (4). Chronic Kidney Disease: In controlled trials, patients experienced greater risks for -----------------------WARNINGS AND PRECAUTIONS------------------------ death, serious adverse cardiovascular reactions, and stroke Increased Mortality, Myocardial Infarction, Stroke, and when administered erythropoiesis-stimulating agents (ESAs) Thromboembolism: Using ESAs to target a hemoglobin level of to target a hemoglobin level of greater than 11 g/dL (5.1). greater than 11 g/dL increases the risk of serious adverse No trial has identified a hemoglobin target level, ESA dose, or cardiovascular reactions and has not been shown to provide dosing strategy that does not increase these risks (5.1). additional benefits (5.1). -
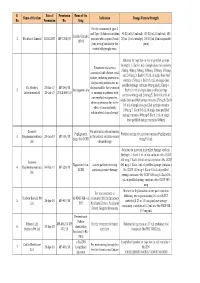
Final List of R-DNA Based Drugs Approved in the Country.Xlsx
S. Date of Permission Name of the Name of the firm Indication Dosage Form & Strength No. Permission No. Drug For the treatment of type -I and Type -II diabetes mellitus 40 IU/ml (10 ml vial), 100 IU/ml (10 ml vial), 100 Insulin Glargine 1 Wockhardt Limited 22.02.2007 MF-7206/07 patients who required basal IU/ml (3 ml cartridge), 100 IU/ml (3 ml disposable 100 IU (long acting) insulin for the pens) control of hyperglycemia. Solution for injection in vial or prefilled syringe Strength: 1. Each 1 mL of single dose vial contains Treatment of anaemia 25mcg, 40mcg, 60mcg, 100mcg, 200mcg, 300mcg associated with chronic renal and 500mcg 2. Each 0.75 mL of single dose vial failure, including patients on contains 150mcg 3. Each 0.3 mL of single dose dialysis and patients not on prefilled syringe contains 60mcg and 150mcg 4. Dr. Reddy's 23-Mar-10 MF-246/10 dialysis and for the treatment 2 Darbepoetin alfa Each 0.4 mL of single dose prefilled syringe Laboratories Ltd. 20-Jul-10 BULK-668/10 of anaemia in patients with contains 40mcg and 200mcg 5. Each 0.42 mL of non-myeloid malignancies, single dose prefilled syringe contains 25mcg 6. Each where anaemia is due to the 0.5 mL of single dose prefilled syringe contains effect of concomitantly 100mcg 7. Each 0.6 mL of single dose prefilled administered chemotherapy syringe contains 300mcg 8. Each 1 mL of single dose prefilled syringe contains 500mcg Gennova For prevention of neutropenia Pegfilgrastim Prefilled syringe for injection contains Pegfilgrastim 3 Biopharmaceuticals 29-Jan-10 MF-104/10 in the patient receiving cancer (peg-r-hu-GCSF) 6 mg/0.6 mL Ltd. -

Presentation Title
The COMMANDS trial: a phase 3 study of the efficacy and safety of luspatercept versus epoetin alfa for the treatment of anemia due to Revised International Prognostic Scoring System Very Low-, Low-, or Intermediate-risk myelodysplastic syndromes in erythropoiesis stimulating agent-naive patients who require red blood cell transfusions Matteo Della Porta,1,2 Uwe Platzbecker,3 Valeria Santini,4 Guillermo Garcia-Manero,5 Rami S. Komrokji,6 Rodrigo Ito,7 Pierre Fenaux8 1Cancer Center IRCCS Humanitas Research Hospital, Milan, Italy; 2Department of Biomedical Sciences, Humanitas University, Milan, Italy; 3Medical Clinic and Policlinic 1, Hematology and Cellular Therapy, University Hospital Leipzig, Leipzig, Germany; 4Azienda Ospedaliero-Universitaria Careggi, University of Florence, Florence, Italy; 5Department of Leukemia, University of Texas MD Anderson Cancer Center, Houston, TX; 6Moffitt Cancer Center, Tampa, FL; 7Bristol Myers Squibb, Princeton, NJ; 8Service d'Hématologie Séniors, Hôpital Saint-Louis, Université Paris 7, Paris, France Presentation 2198 Presenting author disclosures M.D.P.: no conflicts of interest to disclose. 2 Introduction and objectives Introduction • Studies of epoetin alfa and darbepoetin alfa have demonstrated efficacy among patients with LR-MDS, but the patient population in which a clinically significant effect is observed may be limited1,2 • Luspatercept, a first-in-class erythroid maturation agent with a mechanism of action distinct from ESAs,3 is approved by the US FDA for the treatment of anemia failing an -

MEDICATION GUIDE PROCRIT® (PRO′−KRIT) (Epoetin Alfa)
MEDICATION GUIDE PROCRIT® (PRO′−KRIT) (epoetin alfa) Read this Medication Guide: • before you start PROCRIT. • if you are told by your healthcare provider that there is new information about PROCRIT. • if you are told by your healthcare provider that you may inject PROCRIT at home, read this Medication Guide each time you receive a new supply of medicine. This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or your treatment. Talk with your healthcare provider regularly about the use of PROCRIT and ask if there is new information about PROCRIT. What is the most important information I should know about PROCRIT? Using PROCRIT can lead to death or other serious side effects. For patients with cancer: Your healthcare provider has received special training through the ESA APPRISE Oncology Program in order to prescribe PROCRIT. Before you can begin to receive PROCRIT, you must sign the patient-healthcare provider acknowledgment form. When you sign this form, you are stating that your healthcare provider talked with you about the risks of taking PROCRIT. These risks include that your tumor may grow faster and you may die sooner if you choose to take PROCRIT. You should talk with your healthcare provider about: • Why PROCRIT treatment is being prescribed for you. • What are the chances you will get red blood cell transfusions if you do not take PROCRIT. • What are the chances you will get red blood cell transfusions even if you take PROCRIT. • How taking PROCRIT may affect the success of your cancer treatment. -

Epoetin Alfa)
MEDICATION GUIDE PROCRIT® (PRO′−KRIT) (epoetin alfa) Read this Medication Guide before you start PROCRIT, each time you refill your prescription, and if you are told by your healthcare provider that there is new information about PROCRIT. This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or your treatment. Talk with your healthcare provider regularly about the use of PROCRIT and ask if there is new information about PROCRIT . What is the most important information I should know about PROCRIT ? Using PROCRIT can lead to death or other serious side effects. Patients with cancer: Your healthcare provider has received special training through the ESA APPRISE Oncology Program in order to prescribe PROCRIT. Before you can begin to receive PROCRIT, you must sign the ESA APPRISE Oncology Patient and Healthcare Professional (HCP) Acknowledgement Form to document that your healthcare provider discussed the risks of PROCRIT with you. When you sign this form, you are stating that you are aware of the risks associated with use of PROCRIT. These risks include that your tumor may grow faster and you may die sooner when PROCRIT is used experimentally to try to raise your hemoglobin beyond the amount needed to avoid red blood cell transfusion or if you are not getting strong doses of chemotherapy. It is not known whether these risks exist when PROCRIT is given according to the FDA-approved directions for use. You should discuss with your doctor: • Why PROCRIT treatment is being prescribed. • What are the chances you will get red blood cell transfusions if you do not take PROCRIT .